Evaluation of the Oxidative Stress in Chronic Alcoholics
Mamta Singh1, Seema Gupta2, Udita Singhal3, Rajesh Pandey4, S.K. Aggarwal5
1 Research Scholar, Department of Biochemistry, NIMS Medical College (NIMS University), Jaipur, Rajasthan, India.
2 Assistant Professor, Department of Biochemistry, Government Medical College, Haldwani, Uttarakhand, India.
3 Senior Lecturer, Department of Pathology, PDM Dental College and Research Institute, Bahadurgarh, Haryana, India.
4 Associate Professor, Department of Biochemistry, M. M. Institute of Medical Sciences and Research, Mullana, Ambala, Haryana, India.
5 Professor and Head, Department of Biochemistry, M. M. Medical College and Hospital, M. M. University, Kumarhatti, Solan, H.P., India
NAME, ADRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. S.K. Aggarwal, Professor and Head, Department of Biochemistry, M. M. Medical College and Hospital, M. M. University, Kumarhatti, Solan, H.P., India.
Phone: 09991100739,
E-mail: drsurinderkaggarwal@gmail.com
Aim: The present study was conducted to assess the activity of Gamma Glutamyl Transferase (GGT) and its association with oxidative stress in alcoholics.
Method: Sixty male alcoholics with a history of alcohol abuse for more than five years were the subjects of this study. Twenty healthy male volunteers who matched in age and the socio-economic status, served as the control subjects.
Results: GGT, reduced glutathione (GSH, a key intra-cellular antioxidant) and malondialdehyde (MDA, a marker of the oxidative stress) were assayed in the plasma of the two groups, and the results were statistically analyzed. The activity of the plasma GGT, known as a marker of Alcoholic Liver Disease (ALD); was significantly higher in the alcoholics as compared to that in the healthy controls.
Conclusion: There was a significant positive correlation between the enzyme activity and the plasma levels of MDA and this indicated that there was an increased release of this enzyme with enhanced oxidative damage, due to the generation of oxygen free radicals in the study group. There was a significantly increased level of MDA and a decrease in the level of GSH in the alcoholics as compared to those in the controls. Significant negative correlations between GGT and GSH, and between MDA and GSH were observed. The present study demonstrates that alcoholics have a compromised antioxidant defense system.
Alcoholic liver disease, Gamma Glutamyl Transferase (GGT), Malondialdehyde (MDA), Glutathione (GSH), Oxidative stress
Introduction
Alcohol (ethanol) is primarily metabolized in the liver [1]. It is vulnerable to an oxidative stress induced injury due to the generation of reactive oxygen metabolites during the course of the alcohol metabolism and because of this, Alcoholic Liver Disease (ALD) has become a major cause of morbidity and mortality in India [2–3]. The quantity and the type of alcohol which is ingested is the most important risk factor for the development of ALD. An alcohol mediated increased production of pro-oxidants, as well as adecrease in the antioxidant defense systems (enzymatic and non-enzymatic) in the liver may contribute to the development of ALD. An imbalance between the pro-oxidants and the antioxidants leads to oxidative stress which is characterised by escalating the cell damage [4].
Gamma Glutamyl Transferase (GGT; E.C.2.3.2.2) is an enzyme which is derived from the plasma membrane of hepatocytes and its activity has been widely accepted as a biomarker of ALD. Its usefulness as a marker is based on the pharmacological effects of ethanol on the liver; hence, it shows different characteristics in the patients without liver disease as compared to those in the patients with liver disease [5]. GGT is a heterodimeric, membrane-bound glycoprotein [6] which catalyzes the transfer of the gamma glutamyl moiety of glutathione to various peptide acceptors [7]. It has been shown to play an important role in the detoxification of carcinogens and xenobiotics [8]. During the process of the detoxification of xenobiotics, which include alcohol, free radicals are generated through the Microsomal Ethanol Oxidising System (MEOS) as well as through leakage from the mitochondrial Electron Transport Chain (ETC) [9]. Reduced Glutathione (GSH) is one of the frontline non-enzymatic intracellular antioxidants, which safeguards against the oxidative insult. Malondialdehyde (MDA) is a product and hence a marker of the oxidative stress-mediated lipid peroxidation.
The purpose of this study was to determine the activity of GGT and its association with oxidative stress in ALD and this was achieved by measuring the levels of GSH and MDA in chronic alcoholics.
Material and Methods
This study was conducted in the Department of Biochemistry, Medical College and Hospital, NIMS University, Jaipur, Rajasthan, India. The study group comprised of 60 male patients with alcoholic liver disease, who had a history of alcohol intake of more than five years, with a continued daily intake of 80–160g of alcohol. Twenty healthy male volunteers who matched in age and the socio–economic status served as the controls. Only those patients who suffered from jaundice, cirrhosis or chronic liver disease which were caused by alcohol intake were included in the study. The Institutional Ethics Committee approved the study. Before their participation, the volunteers were fully informed of the nature and purpose of the study and a written consent was obtained from each. The patients were subjected to detailed clinical examinations and laboratory investigations. Blood was collected aseptically by venepuncture from the antecubital vein of the patients and the controls into EDTA tubes and the plasma was separated by centrifuging the blood at 1000 rpm for 15 minutes. The exposure of the blood to air was minimised to avoid the oxidation of GSH. The plasma which was obtained was used for the determination of the GGT activity by the method of Szasz et al., [10]. The levels of GSH and MDA were estimated by the methods which were described by Beutler et al., [11] and Buege et al., respectively [12]. The routine liver function tests (ALT and AST) were performed on a multichannel analyzer by using standard commercial kits. All the reagents and the chemicals which were used in this study were obtained from Sigma Chemicals Co., USA. The data which was obtained was subjected to statistical analysis, for evaluating the differences in the mean values of the parameters in the two groups. The statistical significance was determined by using the Student’s ‘t’-test for the unpaired data, the values of significance were evaluated on the basis of the ‘p’-values and the difference was considered as significant at a p value of <0.05. The data was also analyzed for the correlation coefficient amongst the parameters.
Results
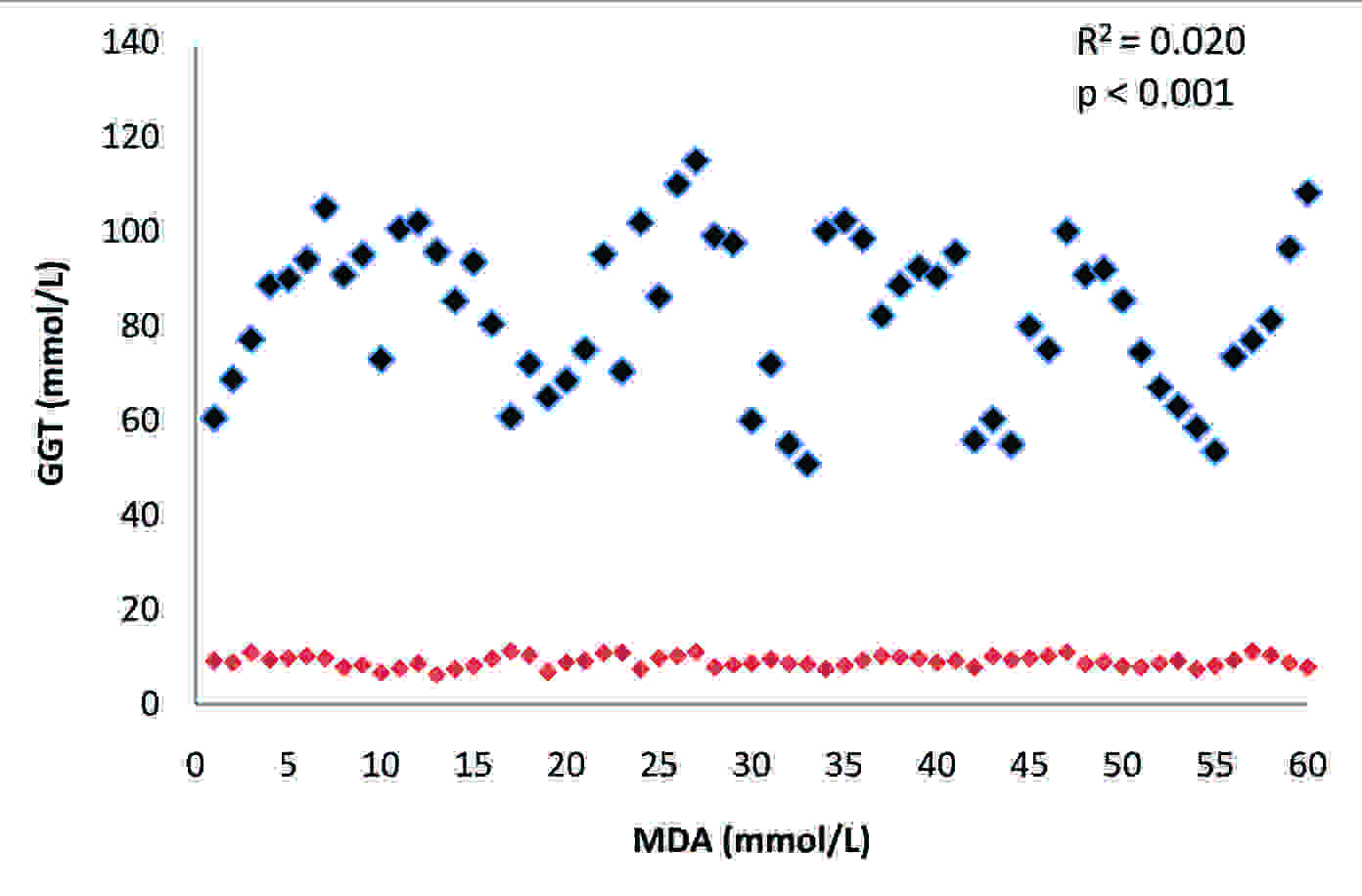
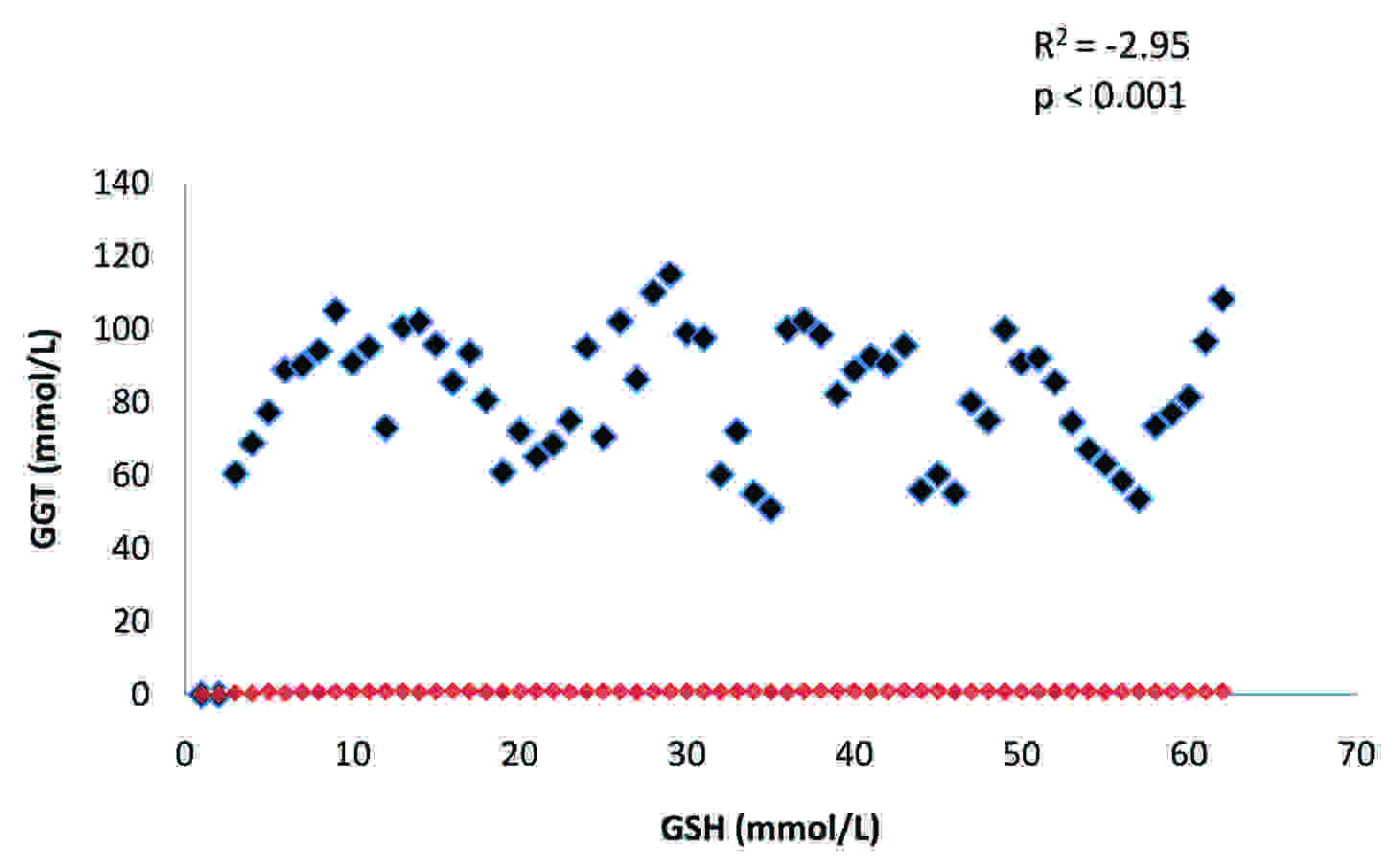
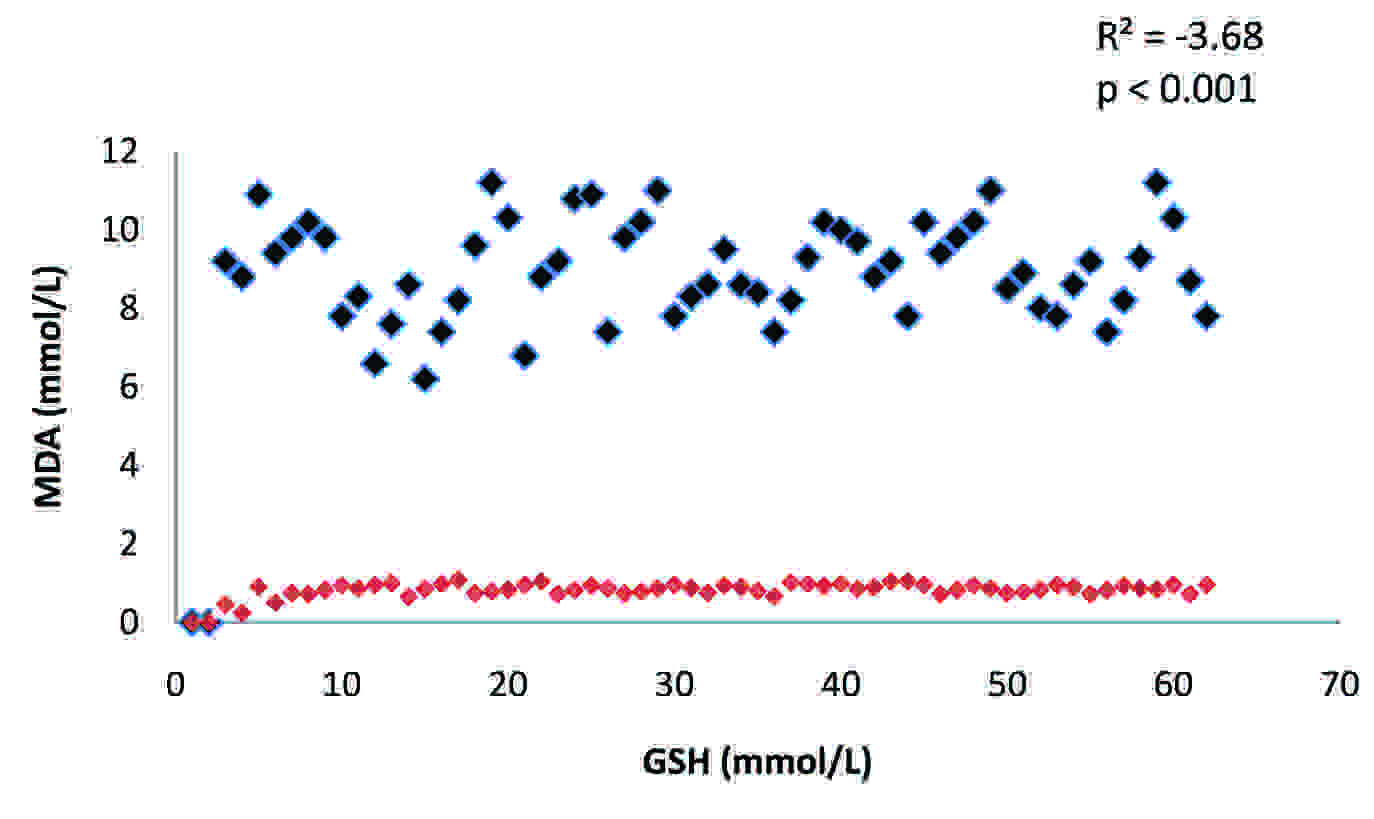
The activity of plasma GGT and the levels of MDA and GSH in the two groups have been presented in [Table/Fig-1]. There was a highly significant difference (p<0.001) in the mean values of the plasma GGT of these two groups. There was a substantial increase in the activity of this enzyme in alcoholics as compared to that in the controls. The activity of this enzyme showed a significant positive correlation (R2: 0.02) with MDA [Table/Fig-2], whereas, it showed a significant negative correlation (R2: -2.95) with GSH [Table/Fig-3] in alcoholics. A significant increase (p<0.001) in the level of MDA was observed in the study group as compared to that in the controls and its levels were significantly negatively correlated (R2: -3.68) to the concentration of GSH [Table/Fig-4].The concentration of GSH was significantly low (p<0.001) in the alcoholics as compared to that in the controls.
Plasma GGT, GSH and MDA in healthy controls and alcoholic patients.
| Parameter | Group I (healthy controls, N=20) | Group II (alcoholics, N=60) |
|---|
| Age (years) | 43.25 ± 4.62 | 48.61 ± 6.27 |
| BMI (kg/m2) | 20.45 ± 0.55 | 17.88 ±1.20 |
| Plasma Enzymes | | |
| • AST (IU/L) | 20.50 ± 5.41 | 63.6 ± 8.7 |
| • ALT (IU/L) | 25.00 ±5.50 | 39.1 ± 5.2 |
| • AST/ALT | 0.8 ± 0.2 | 2.86 ± 0.86* |
| • ALP (IU/L) | 4.90 ± 2.05 | 30.25±3.10 |
| • GGT (IU/L) | 10.2 ± 3.5 | 82.54± 16.42* |
| Plasma proteins | | |
| • Total (g/dl) | 7.184±0.57 | 5.08±0.67 |
| • Albumin (g/dl) | 4.46±0.56 | 3.12±0.65 |
| • A:G | 1.73±0.43 | 1.85±0.75 |
| Plasma MDA (mmol/L) | 3.61±0.63 | 9.02±1.21* |
| Plasma GSH (mmol/L) | 1.46± 1.54 | 0.85±0.15* |
p value: < 0.001; * Group I v/s Group II
Correlation between plasma GGT and MDA in alcoholics
Correlation between plasma GGT and GSH in alcoholics
Correlation between plasma MDA and GSH in alcoholics
Discussion
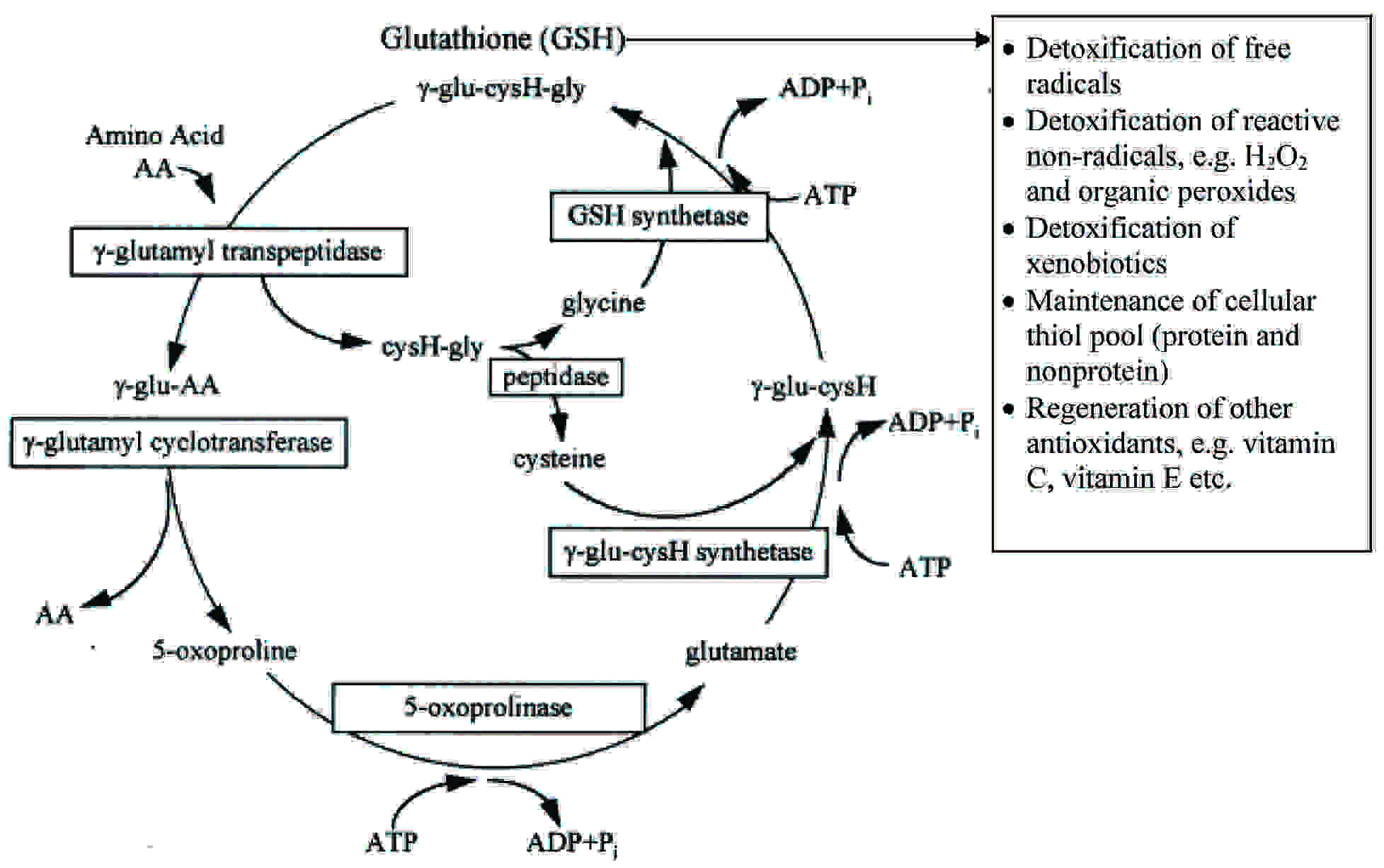
Gamma Glutamyl Transferase (GGT) is an enzyme which is involved in the transfer of the γ-glutamyl residue from the γ-glutamyl peptides to the amino acids. [13] In most of the biological systems, GSH serves as the γ-glutamyl donor [3]. On the other hand, GGT is also involved in the synthesis of GSH [14]. The intra-cellular GSH level depends upon the equilibrium between the processes, during which it is consumed and on its biosynthesis, which, in turn, is limited by the availability of cysteine. The metabolism of GSH is closely connected to Meister’s γ-glutamyl cycle [Table/Fig-5], in which a pivotal role is played by the membrane-bound GGT [15]. This enzyme hydrolyzes GSH to its amino acid components, among which cysteine is used for the intracellular GSH resynthesis [16]. Normally, due to the cysteine toxicity, the physiological level of this amino acid in the cells is very low, and in the plasma, cysteine occurs mainly in the form of a disulfide, i.e. cystine. Consequently, the importance of the γ-glutamyl cycle lies in the recovery and the delivery of cysteine. The availability of cysteine is necessary for the biosynthesis of cellular glutathione, the most important intracellular antioxidant, which depends upon the GGT activity; hence, this enzyme plays an important role in the antioxidant defense system [17].
Synthesis of glutathione (GSH) via the γ-glutamyl cycle (Meister’s cycle) and antioxidant functions of GSH
The plasma GGT activity has been widely used as an index of the liver dysfunction and the alcohol intake. The conditions that increase the plasma GGT, such as obstructive liver disease, high alcohol consumption and the use of drugs, lead to an increased free radical production and the threat of a GSH depletion. Further, the products of the GGT reaction may themselves lead to an increased free radical production. In the present study, a significant increase in the plasma GGT was observed in the patients with ALD, as compared to that in the healthy controls [Table/Fig-1]. The results were in accordance with those of many prospective studies, which demonstrated a strong relationship between the GGT activity and the incidence of ALD [18–20]. Besides alcohol, however, the GGT activity has been found to be raised by multiple factors, which include aging, the smoking habits [21,22] and the exposure to xenobiotics [23]. In our study, age and the BMI were ruled out as the confounders of the plasma GGT difference between non-drinkers and age-matched alcoholics, because the means of age and the BMI did not differ significantly between the two groups. Chronic alcohol consumption results in the proliferation of the smooth endoplasmic reticulum (the site of the intracellular GGT activity) and the induction of the liver derived enzymes, which include GGT [24,25]. This might be responsible for the increased plasma GGT activity in chronic alcoholics. The gradual effect of alcohol on the GGT enzyme induction has been indicated in recent studies, which may be initiated at rather low levels of the ethanol intake [26].
Further, an oxidative stress induced damage to the membrane of the cells which line the bile canaliculi (cells which are rich in GGT) may also contribute to the observed increase in the activity of this enzyme in plasma. The overindulgence in drinking (more than two standard drinks per day over a long period of time) may be associated with a significant elevation in the risk of an ironoverload in the tissue [27]. The deposition of excess iron in the hepatic tissue, is in turn, an important secondary risk factor for the development of ALD. In experimental animals, iron and alcohol have been shown to act in a synergistic manner to enhance the lipid peroxidation, leading to the formation of MDA and liver injuries [28,29].
GSH plays a significant role in the detoxification of the xenobiotics and in the maintenance of the redox status of the cells [Table/Fig-6] [30]. A decline in the cellular GSH level is indicative of an ethanol-induced oxidative stress, which is characterized by the generation of toxic acetaldehyde and other reactive molecules in the cell. The observed increase in the GGT activity is likely to be a defensive response, for detoxifying the toxic metabolites which are produced in the course of the ethanol metabolism [31]. The GSH depletion which was observed in the present study [Table/Fig-1] can be caused by acetaldehyde accumulation [32], and increased utilization by the glutathione-S-transferases as well as GGT, the enzymes which are involved in the detoxification of xenobiotics. Moreover, a reduced GSH synthesis could also be a contributory factor for the GSH depletion, as it has been documented in cirrhotic livers [32].
Oxidative stress as a plausible link between chronic alcoholism and alcoholic liver disease (ALD)
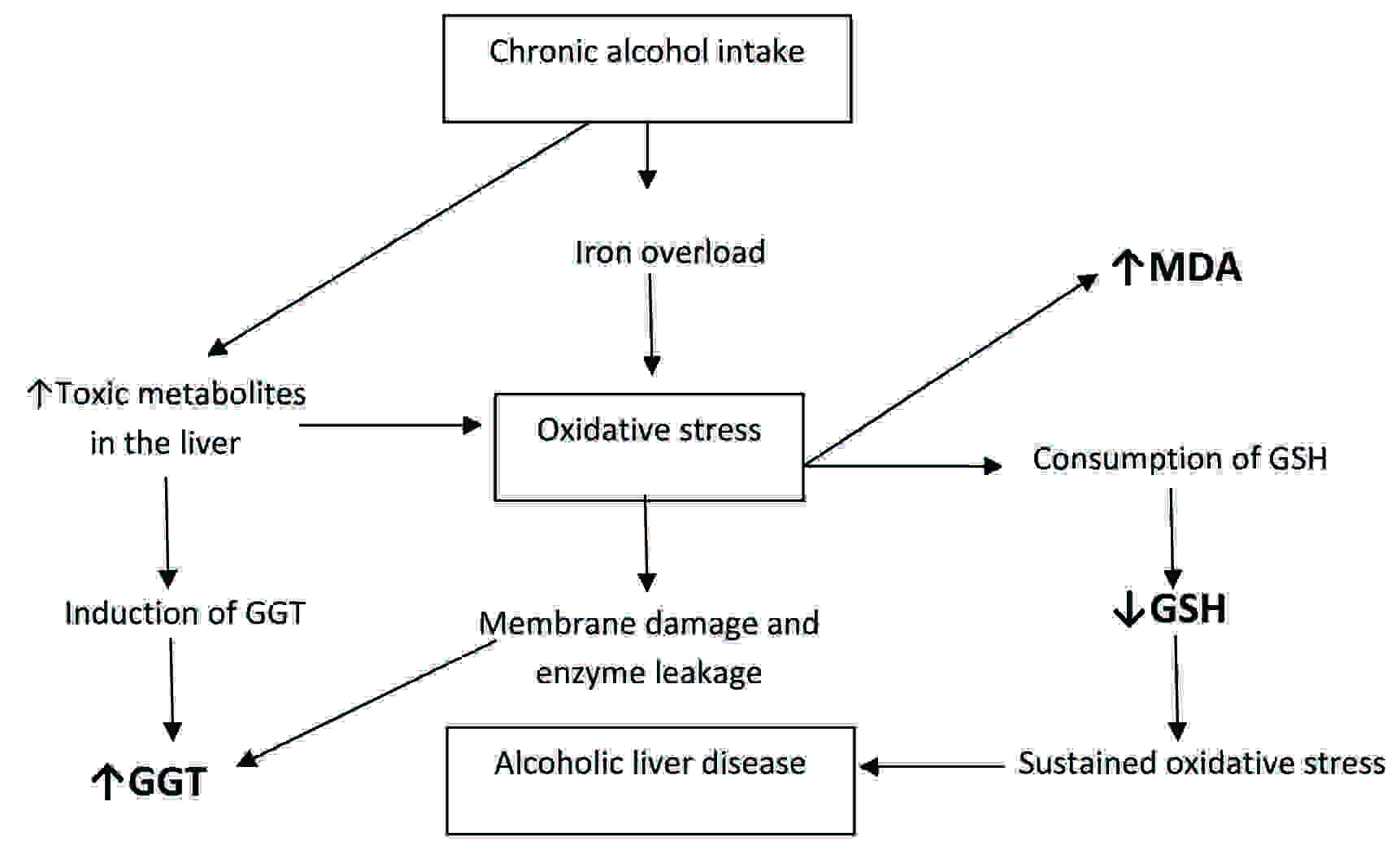
Malondialdehyde, an easily detectable biomarker of oxidative stress, is produced as a result of the oxidative stress-induced lipid peroxidation. In the present study, the levels of plasma MDA in the alcoholics were significantly increased, as compared to those in the healthy controls [Table/Fig-1], which is in agreement with the findings of many studies [31,32]. In addition, MDA showed a positive significant correlation with the GGT levels [Table/Fig-2]. It could be linked to the generation of free radicals by multiple mechanisms, as has been discussed above and the induction of GGT. The main pathway for the alcohol metabolism involves the enzyme, alcohol dehydrogenase [33,34], which metabolizes alcohol into toxic acetaldehyde, whose interaction with the cell proteins and the lipids can result in free radical generation and cellular damage. High oxygen free radical production, as was evidenced by increased levels of MDA and decreased levels of GSH, support the hypothesis that there is increased oxidative stress in the patients with alcoholic liver disease [35].
Nevertheless, there is accumulating evidence which indicates a possible causative involvement of oxidative stress in the pathophysiology of human ALD.
Conclusion
The present study supports the hypothesis that excessive alcohol intake increases the hepatic oxidative stress. Hence, it is not surprising that the antioxidants, as well as the GSH-precursors, are being explored as a supportive treatment or to prevent further deterioration in ALD. The use of the biological markers of alcohol drinking (GGT), antioxidant depletion (GSH) and hepatic damage (MDA), may be of help for the risk assessment in ALD. A better understanding of the pathophysiological mechanism and the correlation between the alcohol intake, the biological markers, and oxidative stress, is an important issue for the better management of ALD.
p value: < 0.001; * Group I v/s Group II
[1]. Vasudevan DM, Sreekumari S, Iso-enzymes and clinical enzymologyTextbook of Biochemistry (for medical students) 2005 4th editionNew DelhiJaypee Brothers Medical Publishers (P) Ltd:57 [Google Scholar]
[2]. Sherlock S., Alcohol and the liverIn: Sherlock S, ed. Diseases of the liver and the biliary system 1995 6th editionLondonBlackwell Publications:385-403. [Google Scholar]
[3]. Singh RB, Ghosh S, Niaz MA, Rastogi V, Wander GS, Validation of tobacco and alcohol intake for the five city study and a proposed classification for IndiansJ Assoc Physicians India 1998 46:587-91. [Google Scholar]
[4]. Chen Yu H, Chan SP, Lu Ting C, The in vivo deleterious effects of ethanolInt J Sport Nutr Exerc Metab 2009 1:81-86. [Google Scholar]
[5]. Nalpas B, Hispard E, Thepot V, Pot S, Dally S, A comparative study between carbohydrates-deficient transferring and gamma-glutamyl transferase for the diagnosis of excessive drinking in a liver unitJ Hepatol 1997 27:127-35. [Google Scholar]
[6]. Tate SS, Meister A, Subunit structure and isozymic forms of gamma-glutamyl transpeptidaseProc Natl Acad Sci USA 1996 73:2599-603. [Google Scholar]
[7]. Reyes E, Miller WR, Serum gamma-glutamyl transpeptidase as a diagnostic aid in problem drinkersAddict Behav 1980 5:59-65. [Google Scholar]
[8]. Lieber CS, Medical disorders of alcoholismN Engl J Med 1995 333:1058-65. [Google Scholar]
[9]. Lieber CS, Role of oxidative stress and antioxidant therapy in alcoholic and non-alcoholic liver diseasesAdv Pharmacol 1997 38:601-28. [Google Scholar]
[10]. Committee of Enzymes of the Scandinavian Society for clinical chemistry and clinical physiology: Recommended method for the determination of γ-glutamyl transferase in bloodScand J Clin Lab Invest 1976 36:119-25. [Google Scholar]
[11]. Beutler E, Duron O, Kelly BM, Improved method for determination of blood glutathioneJ Lab Clin Med 1963 61:882-8. [Google Scholar]
[12]. Beuge JA, Aust SD, The thiobarbituric acid assayMeth Enzymol 1978 52:306-7. [Google Scholar]
[13]. Johnson-Davis K, McMillin GA, Enzymes. In: Bishop ML, Fody EP, Schoeff LE, editorsClinical Chemistry Techniques, Principles, Correlations 2010 6th editionPhiladelphiaLippincott Williams and Wilkins:300 [Google Scholar]
[14]. Zhang H, Forman HJ, Choi J, Gamma-glutamyl Transpeptidase in glutathione biosynthesisMethods Enzymol 2005 401:468-83. [Google Scholar]
[15]. Meister A, Metabolism and transport of glutathione and other γ-glutamyl compounds. In: Larsson A, Orrenius S, Holmgren A, Mannervik B, editorsFunctions of glutathione: Biochemical, physiological, toxicological and clinical aspects 1983 New YorkRaven press:1-22. [Google Scholar]
[16]. Włodek P, Sokołowska M, Smolenski O, Włodek L, The γ-glutamyl transferase activity and non-protein sulfhydryl compounds levels in rat kidney of different age groupsActa Biochim Pol 2002 49(2):501-07. [Google Scholar]
[17]. Moriya S, Nagata S, Yokoyama H, Expression of gamma-glutamyl transpeptidase mRNA after depletion of glutathione in rat liverAlcohol Alcohol 1994 29(1):107-11. [Google Scholar]
[18]. Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP, Aggarwal SK. The lipid peroxide and antioxidant status in alcoholic liver diseasesIndian J Clin Biochem 2005 20:67-71. [Google Scholar]
[19]. Das SK, Nayak P, Vasudevan DM, Biochemical markers of alcohol consumptionInd J Clin Biochem 2003 18(2):111-118. [Google Scholar]
[20]. Pujar S, Kashinakuti SV, Guruadappa K, Manjula R, Serum MDA, antioxidant vitamins and erythrocytic antioxidant enzymes in chronic alcoholic liver disease - A case control studyAl Ameen J Med Sci 2011 4(4):315-22. [Google Scholar]
[21]. Whitehead TP, Robinson D, Allaway SL, The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in menAnn Clin Biochem 1996 33:530-35. [Google Scholar]
[22]. Kono S, Shinchi K, Imanishi K, Coffee and serum gamma-glutamyl transferase: a study of self-defense officials in JapanAm J Epidemiol 1994 139:723-27. [Google Scholar]
[23]. Lee DH, Blomhoff R, Jacobs DR, Is serum gamma Glutamyl transferase a marker of oxidative stress?Free Radic Res 2004 38:535-39. [Google Scholar]
[24]. Ishii H, Yasuraoka S, Shigeta Y, Takagi S, Kaniya T, Hepatic and intestinal gamma-glutamyl transpeptidase activity; its activation by chronic ethanol administrationLife Sci 1978 23:1393-98. [Google Scholar]
[25]. Whitfield JB, Pounder RE, Neale G, Serum γ-glutamyl transpeptidase activity in liver diseaseGut 1972 13:702-8. [Google Scholar]
[26]. Puuka K, Hietala J, Koivsto H, Anttila P, Bloigu R, Age-related changes on serum GGT activity and the assessment of ethanol intakeAlcohol Alcohol 2006 5(41):522-27. [Google Scholar]
[27]. Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV, The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemiaGastroenterology 2004 126(5):1293-301. [Google Scholar]
[28]. Wu D, Cederbaum AI, Alcohol, oxidative stress, and free radical damageAlcohol Res Health 2003 27:277-84. [Google Scholar]
[29]. Findik HD, Shafer D, Klein E, Timchenko N, Kulaksiz H, Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expressionJ Biol Chem 2006 281:22974-92. [Google Scholar]
[30]. Shen YC, Fan JH, Edenberg HJ, Li TK, Cui YH, Polymorphism of ADH and ALDH genes among four ethnic groups in China and effects upon the risk for alcoholismAlcohol Clin Exp Res 1997 21:1272-77. [Google Scholar]
[31]. Singh M, Aggarwal HK, Aggarwal SK, Significance of the glutathione-S-transferase activity and total thiols status in chronic alcoholicsJ Clin Diagn Res. 2012 6(1):31-33. [Google Scholar]
[32]. Zhang X, Li SY, Brow RA, Ren J, Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stressAlcohol 2004 32:175-86. [Google Scholar]
[33]. Maher JJ, Exploring Alcohol effects on liver functionsAlcohol Health Res World 1997 21:5-12. [Google Scholar]
[34]. Pronko P, Bardina L, Satanovskaya V, Kuzmich A, Zimatkin S, Effects of chronic alcohol consumption on ethanol and acetaldehyde metabolizing systems in rat gastrointestinal tractAlcohol Alcohol 2002 37:229-35. [Google Scholar]
[35]. Shinde A, Ganu J, Naik P, Sawant A, Oxidative stress and anti oxidative status in patients with alcoholic liver DiseaseBio Res 2012 23:105-08. [Google Scholar]