Melasma is an acquired hyper-pigmentary disorder, characterized by irregular light or dark brown macules and patches that occur almost exclusively on sun exposed areas of the skin [1]. There is no specific therapy that is universally effective for patients with melasma. Besides the routine use of broad spectrum sunscreens, various topical therapies which are used for the treatment of melasma are 2-5% hydroquinone, tretinoin cream, adaplene, topical corticosteroids, Kligman’s formula and its modification [2,3]. In addition, topical azelaic acid, kojic acid, vitamin C, arbutin and licorice extract and many newer modalities like LASERs have been used in melasma [2,3].
Chemical peeling is now an established weapon in the therapeutic armamentarium for melasma. Today a plethora of superficial chemical peeling agents are being used in melasma like glycolic acid, salicylic acid, Trichloroacetic Acid (TCA) and Jessner’s peel [4,5]. TCA is a versatile peeling agent, since the peel depth can be varied according to the concentration of TCA solution used. It is commonly used as a superficial to medium depth peel with advantages of being stable, inexpensive, self-neutralizing and without any systemic toxicity [6]. However, TCA have been used as superficial peeling agent in very few studies [7,8], which may be due to the fact that hyperpigmentation is a commonly observed side-effect with TCA especially in darker skin type and in patients of tropical countries having intense sun-exposure [9].
Topical ascorbic acid has been reported to be effective and safe in treatment of melasma by many workers [10-13]. This may be due to the fact that it reduces melanin synthesis [10] and has a photo-protective effect [11]. It has an advantage over sunscreen that it is retained in the epidermis for longer time, while sunscreens are easily removed [12]. Ascorbic acid has an important effect on hyperpigmentation due to its ability to affect the monopherase activity of tyrosinase, thereby reducing melanin synthesis, along with its photoprotective effect and antioxidant effect, has been shown to be beneficial in the treatment of melasma with minimal side effects. Thus, through its effect as tyrosinase inhibitor and being an antioxidant, ascorbic acid might help to improve and maintain the response to TCA peel as well as avoid the development of TCA peel induced hyperpigmentation [14]. So, it would be interesting to combine 20% TCA peel with topical 5% ascorbic acid for enhancing the improvement in melasma and reducing the chances of side effects especially PIH, thereby improving the quality of life and patient’s satisfaction.
However, there is only one study by Soliman et al., in the literature using this combination therapy and more elaborate studies are required to confirm the clinical efficacy and safety with this combination [14]. It will also be fruitful to analyse the improvement in QoL, in terms of reduction in MELASQOL, in melasma patients on using this combination therapy. This prompted us to undertake this study to assess the clinical efficacy and safety of combination of 20% TCA with topical 5% ascorbic acid cream in melasma and its effect on MELASQOL.
Materials and Methods
A 12 weeks, open-labelled, non-blinded, prospective, randomized interventional study was conducted in the Department of Dermatology, from August 2012 to January 2014. The study was approved by Post Graduate Board of Medicine and Allied Sciences, Pt. BD Sharma Post Graduate Institute of Medical sciences.
A total of sixty patients of 18 years and above, with epidermal melasma as determined by Wood’s lamp examination, were included in the study. A detailed history including the duration and extent of the disease, family history, past treatment and aggravating/initiating factors were elicited. Exclusion criteria included patients <18 years, pregnancy, lactating mothers, women on oral contraceptives or hormonal replacement therapy, hypersensitivity to the formulations to be used in the study, photosensitivity, those with any other systemic disease (i.e., history of endocrine disorders) or cutaneous disease (such as active/recurrent herpes simplex, facial warts, molluscum contagiosum, history of hypertropic scar/keloids, active dermatosis of atopic, seborrheic or other eczematous type) and unrealistic expectations. Patient who had taken any other treatment (topical or oral retinoids, 5-fluorouracil, imiquimod, laser, dermabrasion, surgery, radiation, etc.,) on the affected region performed within six months prior to the beginning of the study were also excluded. Verbal and written consent were taken from all the patients.
The patients were distributed into two groups using computerized generated randomization chart with thirty patients in each group. Group 1 (combination group) patients received 20% TCA peeling at two weekly interval in addition to topical 5% Ascorbic acid cream once daily at night time and Group 2 (control group) patients received only 20% TCA peeling at two weekly interval. In both the groups, TCA peel sessions were performed at two weeks interval for a total of 12 weeks (total 6 peeling sessions were done).
Materials Used
A 5% topical ascorbic acid cream: It was prepared by an experienced pharmacist using 50 g of ascorbic acid powder in cold cream base (Rosewater, 200 ml; white bee’s wax, 180 g; borax, 10 g; almond oil, 610 g; and oil of rose, 1 ml) [9].
A 20% TCA peeling solution.
Peel Procedure
A postauricular test peel was performed in all the patients to detect any hypersensitivity to the ingredients of the peeling agents. Peeling was performed using the basic guidelines for chemical peeling [15]. The patient’s face was cleaned with cleansing solution (acetone solution) to remove any debris. Using the standard cotton-tipped applicators, a single coat of TCA solution was applied over forehead first followed by the malar areas and finally the chin area. We then waited for the frost formation after which it was washed off with water followed by drying gently with gauze.
All patients were instructed to apply broad spectrum sunscreen (SPF 15+ or greater than 15) regularly and to avoid sun-exposure. All the patients were examined for any side effects like allergic reactions, hypo or hyperpigmentation, persistent erythema, scaling etc.
Clinical Evaluation
a) Melasma area severity index (MASI): MASI developed by Kimbrough Green et al., was used to assess severity of melasma. It was calculated by single-blinded trained dermatologist in every patient before the onset of treatment at baseline and then after each successive peeling session (i.e., after every two weeks) for 12 weeks [16].
b) MELASQOL: Original MELASQOL questionnaire devised by Balkrishnan et al., was used to objectively evaluate QoL of the melasma patients [17]. MELASQOL was calculated at baseline and after 12 weeks (i.e., at the end of study) and the percentage decrease in MELASQOL score was compared between the two groups.
c) Clinical photographs: Front, right and left views of face of each patient were photographed at baseline and after every two weeks interval with high resolution digital camera in natural light.
Statistical Analysis
All statistical analyses were carried out with Statistical Package for Social Sciences (SPSS) for Microsoft windows 20th version. Descriptive statistics were analysed on the parameters of range, mean±standard deviation, frequencies (number of cases), ratio or percentages whichever was appropriate. The change in mean MASI score within each group was analysed using Wilcoxon signed ranks test. The difference in change in mean MASI scoring and MELASQOL score between the two groups was analysed using Mann-Whitney test. Similarly, the percentage decrease in MASI and percentage decrease in MELASQOL score between the two groups were compared using Independent t-test. The side effects between the two groups were compared using Chi-square test.
All tests were performed at a 5% level of significance, thus an association was significant (S) if the p-value was less than 0.05 and non-significant (NS) if p > 0.05.
Results
Baseline Characteristic of Patient
Baseline demographic details are given in [Table/Fig-1]. Both the groups were comparable at baseline as they were well matched for age, sex, duration of disease, baseline MASI scoring and baseline MELASQOL scores (i.e., p-value > 0.05).
The demographic data of the patients included in the study.
| Parameters | Combination Group | Control Group | p-value |
|---|
| Age (years)Mean±SD; Range | 31.83±5.91 (23-47) | 31.50±5.90 (24-47) | 0.828 |
| Sex ratio (male: female) | 1:14 (Males– 2Female- 28) | 1:14 (Males- 2Females- 28) | 1.00 |
| Duration of disease (years) Mean±SD | 2.22±1.06 | 2.32±1.14 | 0.733 |
| Baseline MASIMean±SD | 23.55±4.61 | 23.61±4.09 | 0.959 |
| Baseline MELASQOLMean±SD | 42.67±9.39 | 42.40±8.71 | 0.970 |
Assessment of Clinical Efficacy
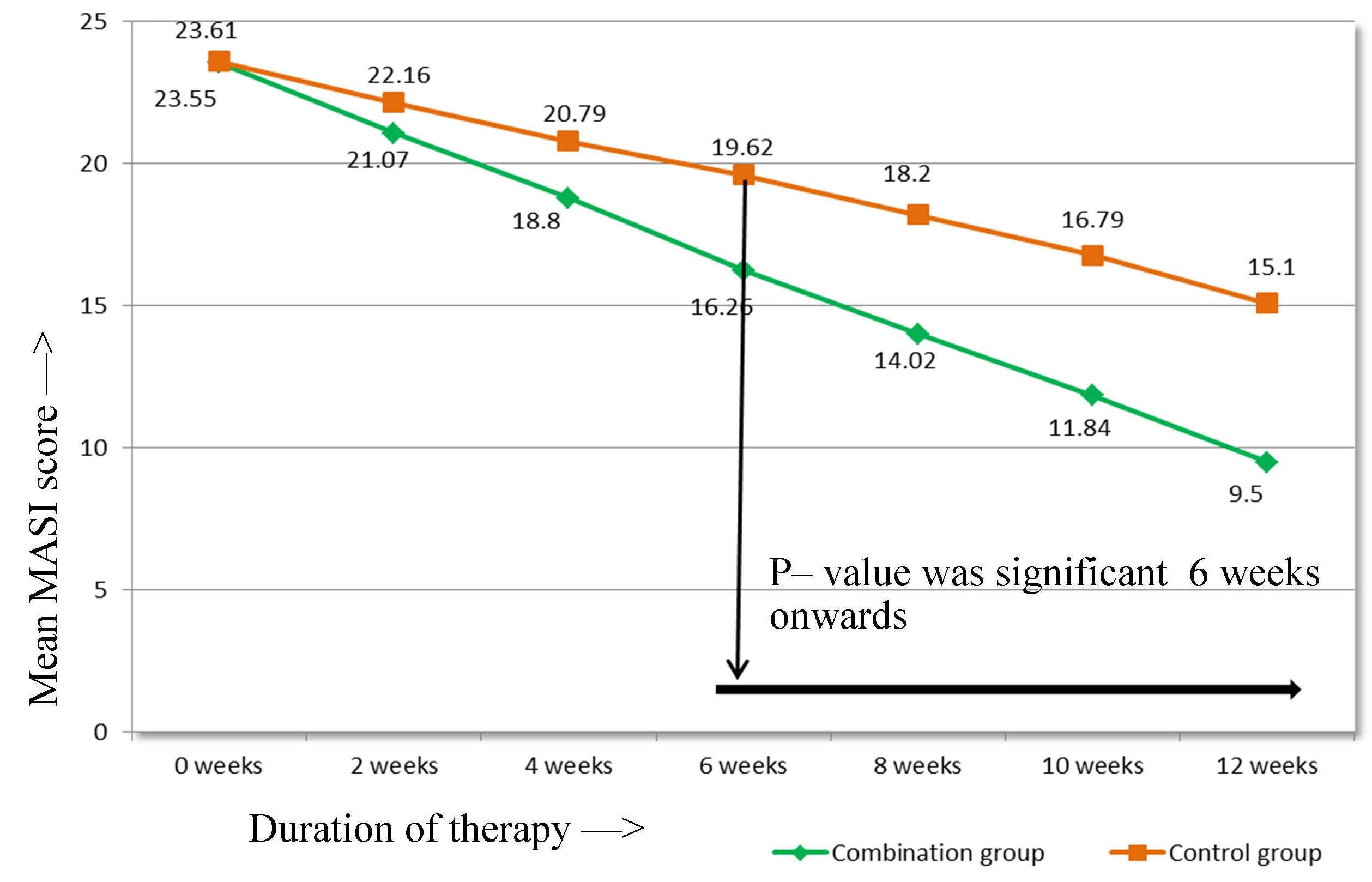
When the reduction in mean MASI score from the baseline was studied in relation to duration of therapy within the group, we found that there was a significant reduction after 2nd week of therapy in both the groups [Table/Fig-2].
Change in MASI within the group throughout the treatment period.
| Variables | Combination Group | p-value (Difference from baseline i.e., 0 week) | Control Group | p-value (Difference from baseline i.e., 0 week) |
|---|
| Baseline MASI(Mean±SD) | 23.55±4.61 | - | 23.6±4.08 | - |
| At 2 weeks | 21.67±4.59 | <0.001 | 22.16±4.04 | <0.001 |
| At 4 weeks | 18.80±4.69 | <0.001 | 20.79±4.12 | <0.001 |
| At 6 weeks | 16.26±4.58 | <0.001 | 19.62±4.20 | <0.001 |
| At 8 weeks | 14.02±4.88 | <0.001 | 18.20±4.23 | <0.001 |
| At 10 weeks | 11.84±5.01 | <0.001 | 16.79±4.31 | <0.001 |
| At 12 weeks | 9.50±5.31 | <0.001 | 15.10±4.44 | <0.001 |
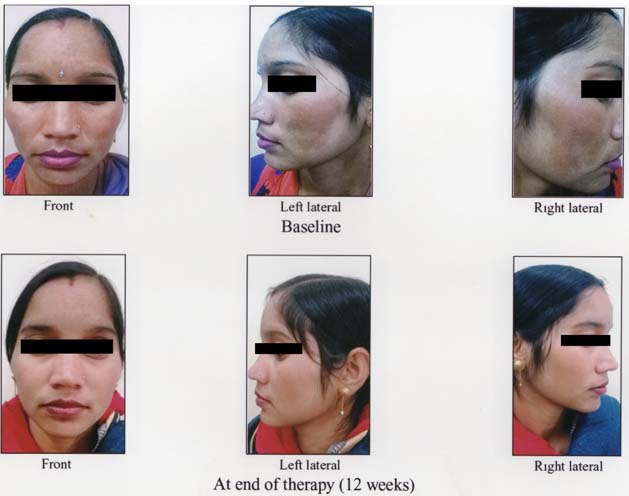
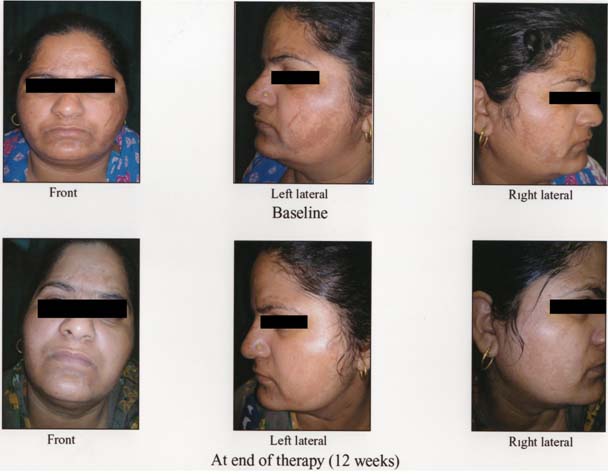
In the combination group, the mean MASI score reduced significantly from the baseline MASI of 23.55±4.61 to 9.50±5.31 at 12 weeks (p-value < 0.001). Similarly, in control group, the mean MASI score reduced significantly from the baseline MASI of 23.613±4.088 to 15.10±4.44 at 12 weeks (p-value <0.001). Thus, aesthetic improvement was seen in all the patients of both the groups [Table/Fig-3,4].
Melasma before and after treatment in combination group.
Melasma before and after treatment in control group.
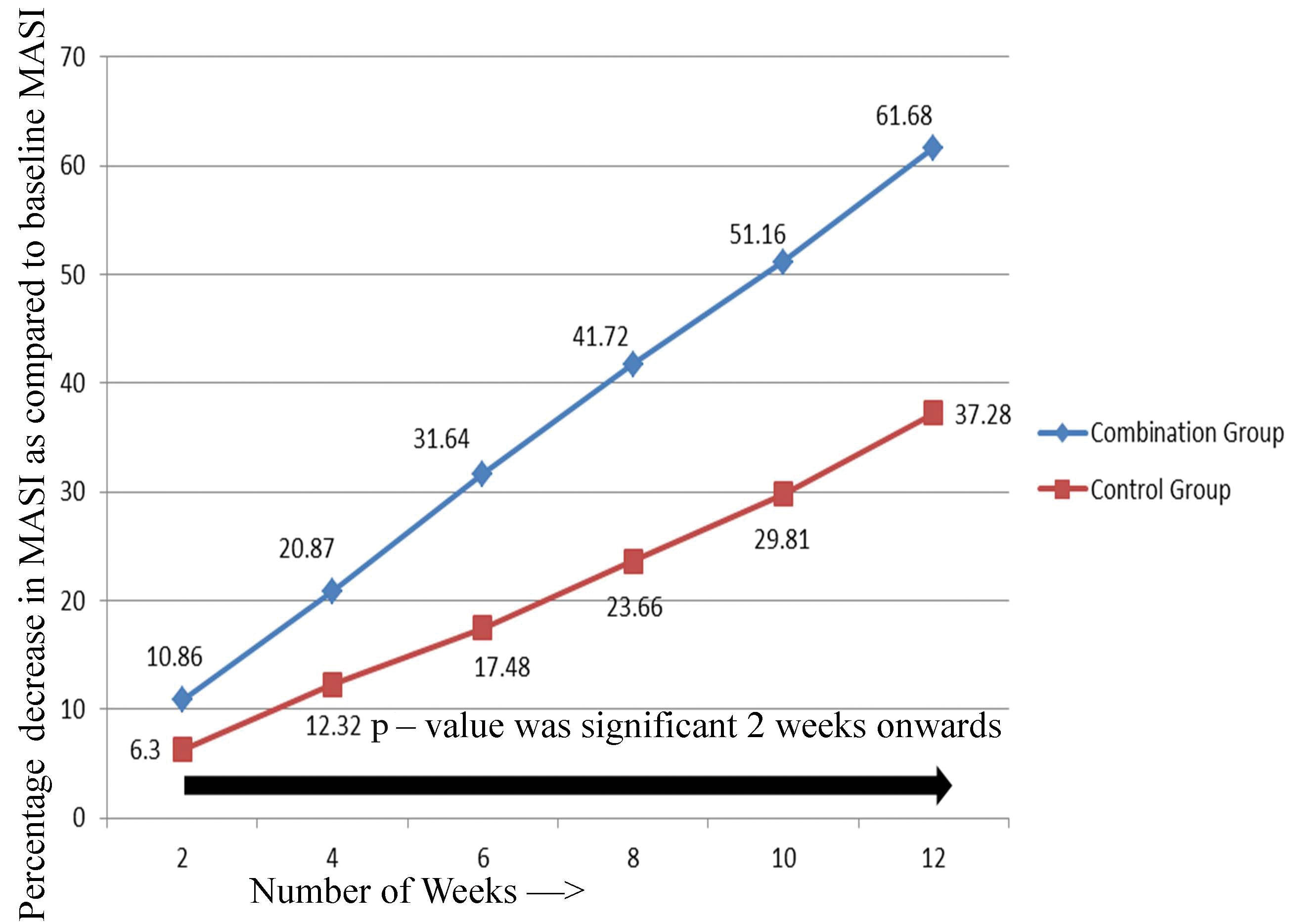
On comparing the reduction in mean MASI score in relation to duration of therapy between the two groups, the combination group showed statistically significant reduction as compared to control group at 6, 8, 10 and 12 weeks of therapy [Table/Fig-5]. When the results were analysed in terms of percentage decrease in MASI from baseline, it was more evident in combination group than control group after 2nd week of therapy [Table/Fig-6]. There was statistically significant difference in percentage decrease in MASI in combination group as compared to control group 2nd week onwards.
Trend of mean MASI score over the weeks.
Percentage decrease in Mean MASI score over the treatment period.
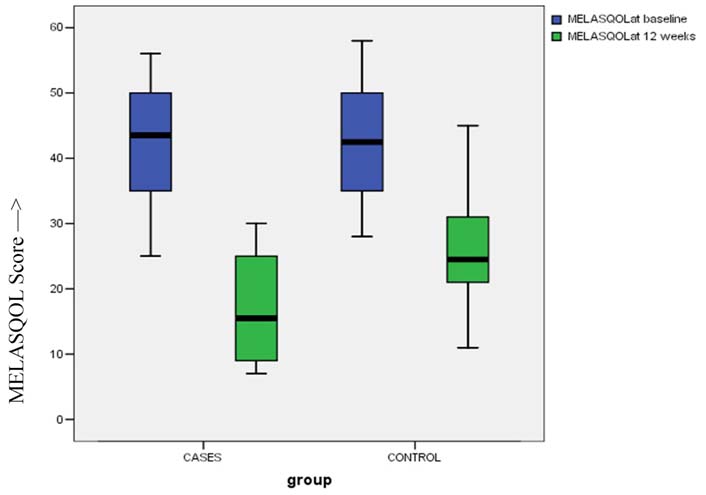
When the mean MELASQOL scores were compared between the two groups at the end of therapy (i.e., 12 weeks), it was found to be lower in combination group (16.60±8.03) as compared to control group (25.90±8.17), with statistically significant difference between them (p-value < 0.001) [Table/Fig-7]. The percentage decrease in MELASQOL score was also found to be statistically significant in combination group (62.36±13.15) as compared to control group (39.87±9.67).
Box and Whiskers Plot: MELASQOL scores before and after therapy.
Safety Evaluation
On assessing the adverse effects, we found that, four patients in both the combination group and control group developed post peel erythema which resolved after giving 1% hydrocortisone cream for one week. Pruritus was not experienced in any patient in combination group but was observed in one patient in control group. Burning and stinging sensation was observed in only four patients in combination group and five patients in control group which resolved with ice cooling, calamine application and use of sunscreens. Post- inflammatory hyperpigmentation was observed in only one patient in combination group but was observed in three patients in control group. Although, the control group was associated with more number of side effects in contrast to the combination group, but the difference between them was not statistically significant (p-value > 0.05).
Discussion
In our study, both combination and control groups showed a statistically significant decrease in mean MASI score within the group as compared to baseline. Thus, skin lightening was observed in all the patients of both the groups. Furthermore, the reduction in mean MASI score and difference in mean percentage decrease in MASI score was significantly higher in combination group as compared to control group at the end of therapy.
This reflects higher efficacy of the combination therapy as compared to TCA peel alone. It corroborates with the findings of Soliman et al., who had also shown a statistically significant decrease in mean MASI and percentage decrease in MASI in the combination group as compared to control group at completion of the study [14].
However, this study had enrolled a very small number of patients i.e., 30 patients with 15 in each group. Thus, clinical studies of combining TCA peel with ascorbic acid cream with larger number of patients and longer time period are lacking in the literature especially in the Indian literature. Our study is a 12 weeks study with larger number of patients i.e., 60.
Patients in either of the groups did not experience any serious adverse effects. Minor adverse effects were seen in 13 patients in control group and 9 patients in combination group. Thus, lower occurrence of side effects was seen in combination group as compared to control group which is in agreement with the previous study by Soliman et al., who had also reported lesser side effects in combination group [14]. However, in Soliman et al., study PIH was not reported in any patient [14]. This might be due to the fact that they had done pre-peel priming with 0.05% tretinoin and 4% hydroquinone two weeks before starting the therapy in patients of both the groups. However, in our study we had observed PIH in one patient in combination group and three in control group, which might be due to absence of pre-peel priming. Pre-peel priming was not done in our study with the intention to observe the actual role of topical ascorbic acid in decreasing the TCA peel induced PIH. Our observation reflects that combining topical ascorbic acid with TCA peel decreases the side-effects and also helps to avoid PIH, which is a potential side effect of TCA peel in dark skinned patients.
In our study, when we analysed the results in terms of MELASQOL, there was a significant decrease in MELASQOL score and percentage decrease in MELASQOL score in combination group as compared to control group at the end of therapy. Thus, a significant decrease in MELASQOL in combination group indicates a better quality of life in combination group patients as compared to control group. A detailed literature scan indicates that there are no studies to highlight the effect of combination of TCA peel with topical ascorbic acid on MELASQOL. Thus, to the best of our knowledge, our study is probably the first study emphasizing that combination of TCA peel and ascorbic acid cream leads to a better quality of life in melasma patients.
Limitation
In our study, there were some limitations: firstly, it was an open–labelled, randomized trial, not a placebo controlled study. Secondly, the study period was relatively short i.e., 12 weeks without any follow-up period. Thus, larger studies with longer follow-up are required to confirm our findings.
Conclusion
Our study concluded that the combination of topical 5% ascorbic acid and 20% TCA peels is a highly effective and well tolerated therapy with lower occurrence of side effects especially PIH and produces a greater improvement in quality of life as compared to 20% TCA peel alone for treatment of melasma.