It is reported that the annual incidence of breast cancer in rural and urban areas in India ranges between 5 and 30 per 100,000 females respectively [1]. Despite the low incidence rate of breast cancer in India compared to western countries, the reported burden of breast cancer with annual incidence being approximately 1,44,000 new cases appears to be high due to the large population [2]. Also, it is observed that in India, majority of breast cancers are diagnosed in advanced stage, attributed to a lack of adequate number of breast screening programs [3]. Now-a-days, accurate diagnosis of breast cancer is made in 99% of cases by “Triple diagnosis” comprising the concurrent use of clinical breast examination, mammography and FNAC [4]. Cytological grading would allow the assessment of the tumour in situ, and it is possible to minimize the morbidity associated with overtreatment of low grade tumours [5]. Nuclear grading is the most important prognostic factor in the cytodiagnosis of carcinoma breast [6]. Cytodiagnosis has reportedly gained importance during the past decade as rapid results are available at a low cost [7]. The National cancer institute recommended tumour grading in FNAC reports of breast carcinoma for prognostication and also insisted that cytological grading should correlate to histopathological grading [8]. Robinson IA et al., Mouriquand J and Pasquier D, Mouriquand J et al., Taniguchi E et al., Fisher ER et al., modification of Black’s nuclear grading, Khan MZ et al., and Howell et al., have proposed various cytological grading systems for breast carcinoma on FNAC [5,9-14]. Studies conducted by some authors have compared and correlated the outcome of these gradings with the SBR method [15]. However, the above mentioned cytological grading methods were never considered as gold standard nor as effective as SBR grading system by both the pathologist and clinicians. Hence the present study was taken up to evaluate and compare two cytological grading systems and to identify the system which has better correlation with the SBR system on excised breast cancer specimens.
Materials and Methods
The present prospective longitudinal study was conducted in the Department of Pathology, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India, from March 2016 to August 2017. Informed consent was obtained from all the patients who were included in the study after approval from the Institutional ethics committee. Female patients clinically diagnosed and confirmed both cytologically and histologically as carcinoma breast were included in the study. Benign breast lesions, recurrent breast cancer, patients having history of chemotherapy or radiotherapy before surgery, when only cytology, histopathology and true-cut biopsy material was available and male patients were excluded. A total of 75 cytologically proven cases of ductal carcinoma of the breast and their corresponding histopathology were included in the study. Haematoxylin and Eosin (H&E), Papanicolaou (PAP) and May-Grunewald-Giemsa (MGG) stained smears were evaluated and the tumour was graded based on the grading system described by Robinson IA et al., [Table/Fig-1] [5] and Mouriquand J and Pasquier D [Table/Fig-2] [9].
Robinson’s criteria for cytological grading [5].
| Cell features | Score I | Score II | Score III |
|---|
| Cell Dissociation | Mostly in clusters | Mixture of singles and clusters | Mostly in single |
| Cell size | 1-2 X RBC | 3-4 X RBC | ≤5 X RBC |
| Cell uniformity | Monomorphic | Mildly pleomorphic | Pleomorphic |
| Nucleoli | Nucleoli Indistinct | Noticeable | Prominent or Pleomorphic |
| Nuclear margin | Smooth | Slightly irregular/folds/grooves | Buds or clefts |
| Chromatin | Vesicular | Granular | Clumped and cleared |
RBC-Red Blood Cell
Mouriquand J and Pasquier D, grading system [9].
| Feature | Score |
|---|
| Cells | Clusters | 0 |
| Isolated | 3 |
| Nuclear features | Anisokaryosis | 2 |
| Large size | 3 |
| Nuclei | Budding | 2 |
| Naked | 3 |
| Hyperchromasia | 2 |
| Hypochromasia | 3 |
| Enlarged nucleoli | Blue | 2 |
| Red | 3 |
| Mitosis | > 3/ per slide | 1 |
| > 6 / per slide | 3 |
Mouriquand J and Pasquier D, grading system [9].
Histological grading was performed on formalin fixed paraffin embedded and H&E stained tissue sections from corresponding mastectomies, lumpectomies and excision biopsies by using SBR method [Table/Fig-3] [15].
Histological grading of breast carcinoma. (Elston and Ellis modification of Scarff- Bloom-Richardson grading) [15].
| Features | Score I | Score II | Score III |
|---|
| Tubule formation | Tubular formation in >75% of the tumour | Tubular formation in 10 to 75% of the tumour | Tubule formation <10% of the tumour |
| Nuclear pleomorphism | Nuclei with minimal variation in size and shape | Nuclei with moderate variation in size and shape | Nuclei with marked variation in size and shape |
| Mitotic count | 0-9 | 10-19 | >20 |
* Field diameter 0.59 mm in Olympus light microscope.
Statistical Analysis
Data were recorded on a Microsoft Excel 2010 spread sheet. A comparison between cytological grading obtained by Robinson’s and Mouriquand’s methods and the histological grading with SBR method was done. Using SPSS version version 16.0, sensitivity, specificity, diagnostic accuracy and concordance and discordance rates were measured by kappa measurement of agreement.
Each feature was assigned a score of 1-3 and the final score obtained by adding up all the scores range between 6 to 18 and translated into final grade as follows: Grade 1=score 6-11, Grade 2=score 12-14, Grade 3=score 15-18.
Grade I: Score <5, Grade II: Score 5-9, Grade III: Score >10
Grade I defined as well differentiated carcinoma, Grade II as carcinoma with pleomorphic tumour cells and Grade III as anaplastic carcinoma [9].
Results
A total of 75 cases, who had undergone FNAC for carcinoma breast, and subsequently underwent surgical resection (excision biopsies-6, lumpectomies-7, mastectomies-62) are included in the study. The breast lumps were more common in the right breast (41 cases-54.66%) compared to left breast (34 cases -45.33%) and most common in the upper and outer compartment (30 cases -40%) followed by central compartment (16 cases-21.33%) [Table/Fig-4]. Out of 75 cases, breast lumps were single in 55 cases and diffuse in 20 cases. Invasive ductal carcinoma of no special type (NST) was reported in 72 cases (96%), mixed ductal and lobular carcinoma, mucinous carcinoma, and metaplastic carcinoma accounted for one case each (1.33%). Age group of the 75 patients ranged from 20 years to 80 years. Majority of the patients were in 4th decade (26 cases, 34.67%) followed by 5th (19 cases, 25.33%) and 6th (11 cases, 14.67%) decade with mean age being 51.61 years.
Percentages distribution of breast carcinoma according to the quadrant involved.
| Quadrant involved | No. of cases | % of cases |
|---|
| Upper and Outer | 30 | 40 |
| Upper and Inner | 8 | 10.66 |
| Lower | 11 | 14.67 |
| Lower and Inner | 6 | 8 |
| Central compartment | 16 | 21.33 |
| Both Upper Outer and Inner | 2 | 2.67 |
| Both Lower Outer and Inner | 2 | 2.67 |
By Robinson’s method out of the 75 cases, 10 cases (13.33%) were grade I, 57cases (76%) were grade II, 8 cases (10.66%) were grade III [Table/Fig-5] According to Mouriquand’s method 14 cases were graded as grade I(18.66%), 54 cases were grade II (72%) and 7 cases were grade III (9.33%) [Table/Fig-6]. Of the 10 grade-I cases reported by Robinson’s method 9 cases were graded as grade I, 1 case was graded as grade II by Mouriquand’s method. Of the 57 cases graded as grade II by Robinson’s method, 50 were graded as grade II,5 were graded as grade I and II were graded as grade III by Mouriquand’s method. Of the 8 cases graded as grade III by Robinson’s method 3 were graded as grade II and remaining 5 as grade III by Mouriquand’s method. The concordance rate between Robinson’s grading and Mouriquand’s grading was 85% (64/75) [Table/Fig-7]. Histological grading was done on surgical specimens according to Elston-Eliss modification of SBR grading system [15]. A total of 23 (30.66%) were grade I, 41(54.66%) were grade II and 11 (14.66%) were grade III [Table/Fig-8]. The concordance rate between Robinson’s grading and SBR grading system was 78.6% (59/75) and the discordance rate was 21.4%(16/75). Similarly, the concordance rate of Mouriquand’s grading with SBR grading system was 66.6% (50/75) and discordance rate was 33.4%(25/75). In order to statistically evaluate which of the two cytological grading methods correspond better to histological grading, grade I cases were considered as low grade and grade II and III together as high grade and the overall sensitivity, specificity, PPV, NPV and diagnostic accuracy of both Robinson’s and Mouriquand’s cytological grading were calculated [Table/Fig-9]. In addition, sensitivity, specificity, PPV, NPV and diagnostic accuracy for individual grades in both Robinson’s and Mouriquand’s cytological grading were calculated [Table/Fig-10].
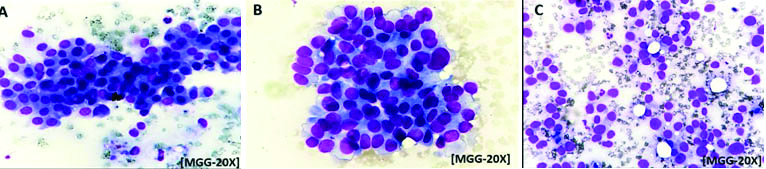
a) Photomicrograph picture shows clusters of monomorphic cells, 1-2 size of RBC, indistinct nucleoli, smooth nuclear margin and vesicular chromatin. Robinson’s cytological grade-I[MGG-20X]; b) Photomicrograph picture shows clusters of cells,3-4 size of RBC, mildly pleomorphic nuclei with irregular margins, granular chromatin and noticeable nucleoli. Robinson’s cytological grade-II (MGG-20X); c) Photomicrograph picture shows individual cells, >5 size of RBC, pleomorphic cells with irregular nuclear margins, clumped chromatin prominent nucleoli and nuclear budding and clefting. Robinson’s cytological grade-III (MGG-20X).
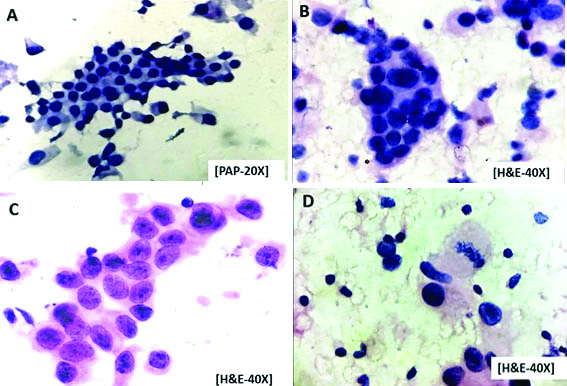
a) Photomicrograph picture showing cluster of cells with mild anisokaryosis, nuclear hypochromasia and no nucleoli. Mouriquand’s cytological grade-I (PAP-20X); b) Photomicrograph picture showing clusters and individual cells with large nuclei, nuclear budding and blue nucleoli. Mouriquand’s cytological grade-II (H&E-40X); c) Photomicrograph picture showing large individual cells and clusters with nuclear hyperchromasia, red nucleoli. Mouriquand’s cytological grade-III (H&E-40X); d) Photomicrograph picture showing large individual cells with nuclear hyperchromasia, prominent nucleoli and mitotic figure. Mouriquand’s cytological grade-III (H&E-40X).
Concordance of two cytological grades.
| Robinson’s grading (n) | Mouriquand’s grading (n) | Concordance n (%) |
|---|
| I | II | III |
|---|
| I | 10 (13.33%) | 9 | 1 | 0 | 64/75 (85%) |
| II | 57 (76%) | 5 | 50 | 2 |
| III | 8 (10.66%) | 0 | 3 | 5 |
| Total | 75 | 14(18%) | 54(72%) | 7(10%) |
Correlation of Nottingham modification of scarff-Bloom Richardson’s method on histopathology with two nuclear grading methods.
| Histopathology Grade | Robinsons grading | Mouriquands grading |
|---|
| I | II | III | I | II | III |
|---|
| I (n=23) | 10 | 13 | 0 | 9 | 14 | 0 |
| II (n=41) | 0 | 41 | 0 | 5 | 35 | 1 |
| III (n=11) | 0 | 3 | 8 | 0 | 5 | 6 |
| Total | 10 | 57 | 8 | 14 | 54 | 7 |
Over all parameters for both grades.
| Statistical parameter | Robinson’s cytological grade | Mouriquand’s cytological grade |
|---|
| TP | 49 | 41 |
| FP | 13 | 15 |
| TN | 10 | 9 |
| FN | 3 | 10 |
| Sensitivity | 94% | 80% |
| Specificity | 43% | 37% |
| PPV | 79% | 73% |
| NPV | 76% | 47% |
| Diagnostic accuracy | 78% | 66% |
TP- true positive, FP-false positive, TN-true negative, FN-false negative, PPV- positive predictive value, NPV- negative predictive value
Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and diagnostic accuracy of Robinson’s (A) and Mouriquand’s [B] cytological grading systems in each grade (n=75).
| Grade | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | Diagnostic Accuracy |
|---|
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
|---|
| I | 10 | 9 | 0 | 5 | 52 | 47 | 13 | 14 | 43% | 39% | 100% | 83% | 100% | 60% | 80% | 77% | 82% | 74% |
| II | 41 | 35 | 16 | 19 | 15 | 16 | 0 | 5 | 100% | 87% | 48% | 45% | 71% | 64% | 100% | 76% | 74% | 53% |
| III | 8 | 6 | 0 | 1 | 64 | 63 | 3 | 5 | 72% | 54% | 100% | 98% | 100% | 85% | 89% | 92% | 96% | 92% |
Discussion
FNAC is a routinely used investigation for rapid diagnosis of breast cancer. The ability to predict the accurate grade on cytology smears would add to the diagnostic value of FNAC, without any additional morbidity or expense for the patients. The purpose of prognostic grading on cytology is to identify high-grade tumours that are more likely to respond to chemotherapy than the low-grade tumours [16]. Assessment of biological aggressiveness by cytological grading without removing the tumour would therefore be valuable. In the present study based on SBR grading system, most of the cases belong to histological grade II, 41 (54.66%), followed by grade I, 23 cases (30.66%) and 11 cases (14.66%) grade III. These results were similar to the results of Younis R et al., and Ahmed I et al., [17,18]. In studies done by Chandanwale SS et al., Chalisa S et al., majority of the cases were grade II (63.8%), (65.51%), followed by grade III (22.4%), (24.14%) and grade I (13.8%), (10.35%) respectively [4,19]. We observed a slight increased preponderance of breast cancer in the right breast, which correlated with study done by Pandey P et al., [20], (63.33%) where as in the study done by Sood N et al., [7], left sided breast was predominantly involved. Location of the tumour in majority of cases in our study was in upper and outer quadrant, which is in correlation with study of Pandey P et al., [20]. Histopathologically 72 cases were invasive ductal carcinoma (NST) and one each, mucinous carcinoma, metaplastic carcinoma and mixed ductal and lobular carcinoma which was similar in distribution to the study done by Agarwal AA et al., [21].
It is observed that one of the limitations in SBR grading is the unequal distribution of cases among the grades with over 50% falling into grade II. This unequal distribution and maximum number of cases falling into grade II was noted in our study similar to other studies. Even though there is a relatively clear prognostic separation between grade I, grade II and grade III cases, grade II cases often overlap with grade I and grade III. Black and speer were the first to introduce nuclear grading [22], which was latter modified by other workers and finally a composite cytonuclear grading was introduced by Robinson IA et al., [5]. Grade II tumours comprised the predominant group both in Robinson’s and Mouriquand’s grading (76% and 72%) respectively which was similar to studies done by Chandanwale SS et al., (56% and 88%), Pandey P et al., (60% and 83.33%), Das AK et al., (46.2% and 69.2%), Wani FA et al., (41.81% and 38.18%), and Arul P and Masilamani S, (74.5% and 59.6%) [4,20,23-25]. Unlike our study Saha K et al., reported grade II tumours (47.4%) as the predominant group in Robinson’s grading and grade III tumours (70.2%) in Mouriquand’s grading [15].
The concordance between the Robinson’s and Mouriquand’s cytological grading systems was 85% in our study which was nearly similar to Wani FA et al., (90.9%) [24]. In the study of Pandey P et al., a concordance of 76.66% was reported [20]. The high concordance in the present study may be due to correlation of grade I and grade II cases between Robinson’s and Mouriquand’s grading systems i.e., 9 out of 10 in grade I and 50 out of 57 in grade II.
In the present study the concordance between Robinson’s cytological grading with SBR histological grading was 78.6% which was almost similar to studies done by Saha K et al., (77.19%), Pandey P et al., (83.33%), Meena SP et al., (83%), Sinha SK et al., (81%), Khan N et al., (88%), Einstein D et al., (77.7%) [15,20,26-29]. Studies done by Robinson IA et al., (57%) and Das AK et al., (71.2%) showed low concordance compared to our study [5,23].
The concordance between Mouriquand’s cytological grading with SBR histological grading in our study was 66.6% which is similar to the studies done by Pandey P et al., (66.6%) and Einstein D et al., (68%) [20,29]. Studies done by Saha K et al., (77.19%) and Das AK et al., (71.2%) showed slightly higher concordance [15,23].
The discordance between Robinson’s cytological grading with SBR histological grading was 21.4% which is nearly similar to studies done by Saha K et al., (22.81%), Pandey P et al., (16.66%) Meena SP et al., (17%), Sinha SK et al., (19%), Khan SK et al., (12%), and Einstein D et al., (22.3%) [15,20,26-29]. Studies done by Robinson IA et al., (39.5%) and Das AK et al., (28.8%) showed relatively high discordance [5,23].
The discordance in the present study was due to grade II tumours (57 cases), of them 13 were downgraded to grade I and three were upgraded to grade III. With these results we infer that Robinson’s cytological grading is a reliable method of grading breast carcinoma in FNAC smears. The reasons for the discordance is due to heterogeneity of tumour, observer subjectivity when assessing nuclear grade and all of them showed only one grade discordance and moreover histological grading was based on the degree of formation of tubules, mitosis and nuclear pleomorphism. Some authors believed that dissociation or clustering reflect tubule formation though it is very difficult to assess tubule formation on FNAC smears [5,9,23].
The discordance between Mouriquand’s cytological with SBR grading was 33.4% which is similar to the study done by Pandey P et al., (33.33%) and Einstein D et al., (32%) [20,29]. Studies done by Saha K et al., (22.81%) and Das AK et al., (28.8%) showed lower discordance compared to the present study [15,23]. The discordance in the present study was observed in all the grades. Among 14 grade I cases, 5 were upgraded to grade II. Among 54 grade II cases, 14 were down grade to grade I and 5 were upgraded to grade III. Among 7 grade III cases, 1 was downgraded to grade II. One of the reasons for discordance with Mouriquand’s grading is the presence of mitosis.
In the present study Robinson’s and Mouriquand’s cytological gradings have sensitivity of 94% and 80% and a low specificity (43% and 37%). which was similar to the study done by Das AK et al., (sensitivity-81.39% and 95.35%) and low specificity [23].
The diagnostic accuracy for Robinson’s and Mouriquand’s cytological gradings in our study was 78% and 66% respectively [Table/Fig-9], which was nearly comparable with Pandey P et al., study (90% and 76.66%) and Das AK et al., (80.76% and 84.6%) [20,23].
In the present study, the kappa values of agreement for Robinson’s and Mouriquand’s cytological gradings were k=0.100 (very good agreement) and k=0.118 (fair agreement) respectively [Table/Fig-11], which are better than kappa values (k=0.28, fair agreement), (k=0.18) respectively shown by Chandanwale SS et al., [30].
Kappa value for Robinson’s [A] and Mouriquand’s [B] grading.
| Grade | Kappa statistic | SE of kappa | 95% of CI Interval | Strength of agreements |
|---|
| A | B | A | B | A | B | A | B |
|---|
| I | 0.516 | 0.331 | 0.107 | 0.118 | 0.307-0.725 | 0.101-0.562 | Moderate | Fair |
| II | 0.516 | 0.341 | 0.093 | 0.102 | 0.333-0.699 | 0.352-0.896 | Moderate | Fair |
| III | 0.820 | 0.624 | 0.100 | 0.139 | 0.623-1.00 | 0.352-0.896 | Very Good | Good |
In the present study, we found that Robinson’s cytological grading correlated better than Mouriquand’s cytological grading with SBR histological grading which is because Robinson’s cytological grading has two more criteria which are cell dissociation and uniformity which are absent in Mouriquand’s cytological grading. The criteria for grading of tumour by Robinson’s method was simple and easily reproducible compared to Mouriquand’s method. Also, Robinson’s method was more specific than Mouriquand’s when SBR grading system was considered as gold standard.
Limitation
Small sample size and low specificity due to variation of cytological features in different areas of tumour on histopathology, which cannot be appreciable on cytology because of limited area of approach.
The present study recommends, the addition of mitotic figures to Robinson’s cytological grading consisting of cellular features like cell size, pleomorphism, cellular dissociation, nuclear features such as nucleoli, nuclear margin and chromatin. Addition of mitotic figures to the cytological grading will increase the specificity, sensitivity and concordance rates.
Conclusion
It was concluded that the cytological grading of breast carcinoma can be done by both Robinson’s and Mouriquand’s cytological grading with slightly variable concordance with histological grading. Pitfalls of cytological grading were over graded by both Robinson’s and Mouriquand’s method with accumulation of cases in grade II. However the Robinson’s cytological grading appears to be slightly superior to Mouriquand’s cytological grading and hence can be considered better for routine cytological grading of breast carcinomas.
RBC-Red Blood Cell
Mouriquand J and Pasquier D, grading system [9].
* Field diameter 0.59 mm in Olympus light microscope.
TP- true positive, FP-false positive, TN-true negative, FN-false negative, PPV- positive predictive value, NPV- negative predictive value