Celiac disease is a heritable, chronic, autoimmune gastrointestinal disorder of the small intestine and is caused by an inappropriate immune response to wheat gluten [1]. Gluten can lead to oxidative stress in CD. Gliadin peptide may increase the production of free radicals including Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and cause oxidative imbalances such as an increase in oxidised and reduced glutathione ratio [1]. It is rich in proline residues and difficult to hydrolyse, hence resistant to digestion [2]. Further, a high concentration of proline can exert toxic effects in CD [1]. Nerve Growth Factor (NGF) is a neurotrophic factor that functions in the regulation of survival and development of neurons [3]. It is also expressed in numbers of immune cells such as thymocytes, spleen mononuclear cells, T-lymphocytes, B-lymphocytes, monocytes and mast cells [4]. NGF is associated with the pathophysiology of different autoimmune diseases, such as rheumatic arthritis, knee joints, multiple sclerosis, lupus erythematosus and allergic encephalomyelitis [3,4].
Prolidase is a metalloproteinase that cuts dipeptides containing OH-proline or proline at the C-terminal end and is involved in collagen metabolism [5]. Its activity has been reported in many chronic inflammatory diseases [6-8]. It has been reported that proline dipeptidase (prolidase) activity decreases in small intestinal mucosal homogenates of children suffering from different degrees of villous damage [9]. Till now, no study is present in the literature regarding the status of SPA and NGF in CD patients. Thus, the present study aimed to evaluate the serum NGF, SPA and oxidative stress in the patients with CD. A correlation of tissue transglutaminase immunoglobulin-A (tTG-IgA) with NGF, SPA and oxidative stress was also evaluated.
Materials and Methods
The present case-control study was completed in the Department of Biochemistry and Department of Paediatrics of Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, India between the September 2011 to August 2016. Ethical clearance for the study was obtained from institutional (IMS-BHU) Human Ethical Committee. Written consent was taken from all the subjects (patients and controls). Patients were recruited from the Outpatient Department (OPD) of Department of Paediatrics and screened out with the association of Department of Gastroenterology and Department of Pathology; while controls were recruited from the Blood Bank of IMS, BHU, Varanasi, India.
Total 92 subjects were selected including 46 patients with CD and 46 healthy children of matched age and gender as cases and controls, respectively. The cases were diagnosed by the expert clinician based on clinical history, physical examination, endoscopic and pathological (tTG-IgA) examinations.
Serum separation: Blood was collected in plane vials. Serum was separated at 3000 rpm for 10 minutes and stored at -80°C until use.
Estimation of beta-NGF
Serum NGF was estimated with Abcam ELISA kit (ab193760) for the quantitative detection of beta-NGF. Final OD of the test was taken at 450 nm and results were calculated with the typical standard curve and represented as pg/mL.
Estimation of Serum Prolidase Activity (SPA)
Serum prolidase activity was measured with the use of the previous standardised method of Verma AK et al., [10], which are solely based on Myara I et al., [7]. Principally, estimation of prolidase activity is based on the estimation of end product (proline) of the enzyme. The end product of enzyme, proline, makes a coloured complex with Chinard’s reagent (containing glacial acetic acid, orthophosphoric acid and ninhydrin). The intensity of colour defines the intensity of enzyme activity. For the estimation of SPA, this colour intensity was measured at 515 nm spectrophotometrically and results were expressed in mmol/minute/L.
Estimation of Tissue Transglutaminase Immunoglobulin-A (tTG-IgA)
Serum tissue Transglutaminase Immunoglobin-A (tTG-IgA) was measured by the use of ELISA kit (Phadia GmbH, batch code-CD V015, 181-05/UK). Serum was diluted with sample diluents (1:100 ratio). All steps of assay were followed strictly as per manufacturer. Final absorbance was taken at 450 nm and final results were expressed in U/mL.
Estimation of Oxidative Stress Parameters
Oxidative stress parameters such as TOS and TAS were estimated with the use of methods developed by Erel in 2005 and 2004, respectively [11,12]. The results of TOS and TAS were represented as mmol H2O2 Equivalent/L and μmol Trolox Equivalent/L respectively.
Computation of Oxidative Stress Index (OSI)
Ratio of TOS to TAS was computed and considered as oxidative stress index [9]:
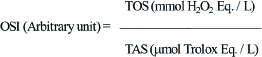
Statistical Analysis
Standard parametric statistical methods, student’s t-test, and Pearson’s correlation were used. A p-value less than 0.05 was considered as significant, however p-value greater than 0.05 was non-significant.
Results
Demographically, the selected 46 patients with CD having 29 boys and 17 girls with mean age 6.24±2.66-year-old; however, 46 healthy controls having 30 boys and 16 girls with mean age 6.58±2.68-year-old [Table/Fig-1].
Status of tTG-IgA, NGF, SPA, and oxidative stress parameters (TOS, TAS and OSI) in the patients with CD and healthy children.
| Parameters | Patients with CD n=46 | Healthy Children n=46 | p-value |
|---|
| Gender (male/female) | 29/17 | 30/16 | - |
| Age (years) | 6.24±2.66 (R=2-12) | 6.58±2.68 (R=2-12) | 0.543 |
| NGF (pg/mL) | 28.62±1.50 | 21.91±3.83 | <0.001* |
| SPA (mmol Min-1L-1) | 177.78±54.21 | 136.01±33.56 | <0.001* |
| tTG-IgA (U/mL) | 161.98±19.69 | 3.80±1.25 | <0.001* |
| TOS (mmol H2O2 Eq/L) | 17.11±1.74 | 11.94±1.26 | <0.001* |
| TAS (μmol trolox Eq/L) | 1.36±0.26 | 2.49±0.56 | <0.001* |
| OSI (Arbitrary unit) | 13.22±3.59 | 5.14±1.63 | <0.001* |
tTG-IgA: Tissue transglutaminase immunoglobulin A; NGF: Beta-nerve growth factor; SPA: Serum prolidase activity; TOS=Total oxidant status; TAS: Total antioxidant status; OSI=Oxidative stress index; n=Number of subjects; Significance p-value are denoted by*
Status of beta-NGF: The status of serum NGF was significantly elevated in the celiac patients when compared to healthy controls (p<0.001, [Table/Fig-1]).
Serum prolidase activity (SPA): According to the nature of results, SPA was significantly elevated in the patients with CD (177.78±54.21 mmol Minute-1L-1) as compared to healthy controls (136.01±33.56 mmol Minute-1L-1) (p<0.001, [Table/Fig-1]).
Tissue transglutaminase-IgA immunoglobulin (tTG-IgA) status: tTG-IgA was significantly elevated in the patients with CD as compared to healthy controls (p<0.001, [Table/Fig-1]).
Total oxidant status (TOS): The status of TOS was significantly elevated in the patients with CD (17.11±1.74 mmol H2O2 Eq/L) as compared to healthy controls (11.94±1.26 mmol H2O2 Eq/L) (p<0.001, [Table/Fig-1]).
Total Antioxidant Status (TAS): According to observed results, TAS was significantly decreased in the patients with CD (1.36±0.26 μmol Trolox Eq/L) than the healthy controls (2.49±0.56 μmol Trolox Eq/L) (p<0.001, [Table/Fig-1]).
Oxidative Stress Index (OSI): Level of OSI was significantly increased in the patients with celiac disease than the healthy children (p<0.001, [Table/Fig-1]).
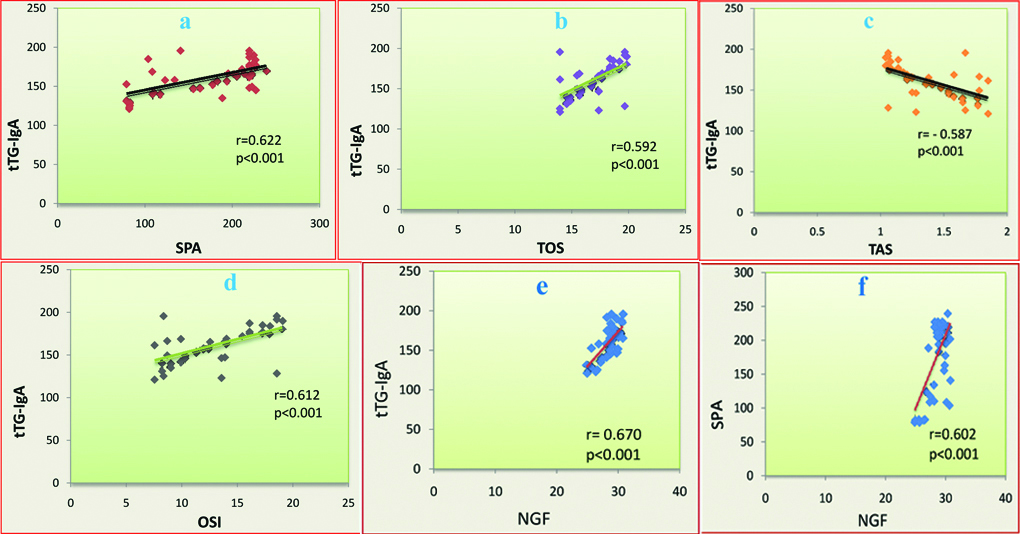
On correlating observations for the patients with celiac disease, tTG-IgA positively and linearly correlated to SPA (r=0.622, p<0.001), TOS (r=0.592, p<0.001) and OSI (r=0.612, p<0.001) which was statistically significant ([Table/Fig-2a,b,d] respectively). However, tTG-IgA negatively and linearly correlated to TAS (r=-0.587, p<0.001) in the patients with CD, which was also statistically significant [Table/Fig-2c]. This is a very interesting observation that tTG-IgA significantly and positively correlated to NGF (r=0.670, p<0.001, [Table/Fig-2e]) in the patients with CD. The elevated status of NGF also significantly correlated with elevated status of SPA (r=0.602, p<0.001, [Table/Fig-2f]) in celiac patients.
Diagrammatic representation of linear correlation between tTG-IgA and SPA, TAS, OSI, and NGF (a-e, respectively) in the patients with celiac disease
(all p<0.001); f: represents linear correlation between NGF and SPA (p<0.001). r=Pearson’s correlation coefficients.
Discussion
The NGF-β is a neurotrophin family member that is involved in cell survival and differentiation during the nervous system development [13]. It is present in different cells of the immune system including B and T lymphocytes, eosinophils, and mast cells, which is also present in immune organs such as thymus, lymph nodes, and spleen. Elevated NGF is associated with an increase in inflammatory response, chronic inflammation, allergic diseases, in infections and autoimmune diseases [3,13]. Elevation of NGF has been reported in lupus erythematosus, systemic sclerosis, multiple sclerosis, allergic encephalomyelitis and rheumatoid arthritis [14]. The events related to stress (either anxiogenic conditions or psychologically induced stress) can also induce the elevation of NGF [3]. Thus, it is seen that elevation of NGF is associated with the chronic inflammation, autoimmune diseases and stress. However, till date the role of NGF in the patients with CD (a chronic inflammatory and autoimmune disease) is unexplored. Therefore, the present study was aimed to evaluate the status of NGF in the patients with CD. In the present study, the status of NGF significantly elevated in the patients with CD as compared to healthy children (p<0.001, [Table/Fig-1]). Thus, it seems that like other chronic and autoimmune diseases, elevated NGF might be associated with the pathophysiology of the celiac disease.
Proline is a special type of amino acid. In the case of plants, proline is accumulated in the response to stress. It is an excellent osmolyte and plays three major roles; as a metal chelator, a signalling molecule and an antioxidative defence molecule during stress. Unfortunately at higher concentration proline can exert toxic effects [15]. Celiac patients are susceptible to the autoimmune reaction of gliadin peptide that is present in wheat and related cereals. Gliadin peptide is composed of high contents of proline. In the case of celiac disease, the oligopeptides of gliadin have resistance to complete digestion and are accumulated in the small intestine. The accumulated concentration of proline-rich peptide can cause a toxic effect on the lumen of small intestine and may be associated to increase in oxidative stress [1]. A study has been demonstrated that a higher concentration of proline can increases the generation of oxygen free radicals and decreases the status of superoxide dismutase enzyme. Therefore, a higher concentration of proline can induce the oxidative stress [16,17]. It is known that prolidase is an enzyme that is involved in proline-rich collagen metabolism. It splits C-terminal gly-L-pro peptide into proline or hydroxyproline [11,12]. The present study reported that serum prolidase activity significantly elevated in the patients with celiac disease as compared to healthy children (p<0.001) [Table/Fig-1]. Thus, it seems that elevated serum prolidase activity may increase the proline concentration in the circulation that might be associated with the increase in oxidative stress in the patients with celiac disease.
Tissue transglutaminase (tTG) or Type 2 transglutaminase (TG2, EC 2.3.2.13) is a multifunctional enzyme belonging to the transglutaminase family [18]. In routine pathology measurement of tTG is frequently used for screening of celiac disease. In the present study, all the patients with celiac disease had the significantly elevated status of tTG-IgA as compared to healthy controls (p<0.001, [Table/Fig-1]). Considering the pathological point of view, tTG can lead to deamination of glutamine to glutamic [19]. tTG can activate different immunological responses (activation of IL-1β, IFN-γ, TNF-α and Th1 cells) [20]. In response to trauma and stress, tTG gets translocated to the plasma membrane where it gets deposited into the Extra Cellular Matrix (ECM) and activates inflammatory response. There is evidence suggesting the involvement of the tTG in oxidative stress via an increase in ROS and decrease in antioxidants levels [20,21]. Thus, it is seen that tTG is associated with increased oxidative stress. But, till now little is known regarding the mechanism by which tTG leads to oxidative stress in the patients with CD. Thus, the present study explored the association of tTG with oxidative stress in the CD. According to the present study, tTG has been positively and linearly correlated to SPA, and TOS, and OSI (all p<0.001, [Table/Fig-2a,b,d]) in the patients with celiac disease. However, tTG has been negatively and linearly correlated to TAS in the celiac patient which is also significant (p<0.001, [Table/Fig-2c]). Thus, it seems that over expression of tTG in the patients with CD can lead to increased oxidative stress and might be associated with the pathogenesis of the disease.
From the above discussion, it is clear that tTG can be associated with the over expression of different immunoglobulin and cytokines. Like tTG, NGF is also involved in T-cell and B-cell activation and immunoglobulin secretion [22,23]. Interferon beta mediates NGF expression [24] and further NGF mediates the progressive fibrosis [25]. Thus, NGF is involved in growth, inflammation and fibrosis. Besides these, a literature survey reported that NGF also evokes transglutaminase-2 induction [26]. Like tTG and NGF, prolidase is also involved in chronic inflammation and fibrosis [5-7]. However, until now, no study is available in the literature regarding the association of tTG, NGF and prolidase enzyme in the celiac patients. In this consequence, the present study explored that the elevation of tTG has been positively correlated to an elevation of NGF and SPA (all p<0.001, [Table/Fig-2a,e]) in the patients with CD. It has been observed that the elevated NGF positively correlated to an elevation of SPA (p<0.001) [Table/Fig-2f] in CD. Thus, it seems that elevated tTG might be associated with elevation of NGF and SPA or vice-versa, and might be associated with the pathogenesis of celiac disease. However, it needs explorative study.
Oxidative stress is a condition created by an elevation of ROS and/or RNS as well as the decrease in efficiency of enzymatic and/or non-enzymatic antioxidants system [27]. ROS and RNS are also associated with different inflammatory cell signalling and chronic inflammation [28]. Characteristically, the CD is associated with small intestine chronic inflammation, elevation of free radicals and activation of nitric oxide synthase [28]. Furthermore, it is known that patients with CD express inducible nitric oxide synthase in the intestinal wall that results in increased levels of nitric oxide. As the nature of nitric oxide, it can react with the super oxide radical which leads to the production of peroxy nitric which is an important oxidising agent [29]. As well, continued production of ROS during the chronic inflammation of celiac intestinal wall can crush the antioxidant defence system leading to more damage to tissue [30]. Thus, it appears that increased oxidative stress of CD patients is due to the increased production of ROS, chronic inflammation and crushed antioxidant defence system. However, in the case of patients with CD, there is a lack of literature regarding the oxidative stress in the term of total oxidant and antioxidant status. According to the present study, TOS and OSI have been significantly elevated in the patients with CD, while TAS has been significantly decreased (all p<0.001, [Table/Fig-1]) and collectively patients with CD have increased oxidative stress. Thus, it seems that increased oxidative stress might be responsible for the pathogenesis of CD via prolonged chronic inflammation and tissue injury.
Limitation
Small sample size was a limitation of study. It was a clinical study, a more explorative study on proteomics as well as molecular study of NGF and SPA in CD is needed for better clarification.
Conclusion
The present study concludes that status of nerve growth Factor, serum prolidase activity, tissue transglutaminase and oxidative stress is significantly elevated in the serum of patients with CD as compared to healthy controls. It also concluded that the elevated tTG significantly correlates to elevated NGF, SPA and oxidative stress in the patients with CD. Thus, it seems that elevated NGF, prolidase enzyme, tTG and oxidative stress might be responsible for the pathogenesis of celiac disease.
tTG-IgA: Tissue transglutaminase immunoglobulin A; NGF: Beta-nerve growth factor; SPA: Serum prolidase activity; TOS=Total oxidant status; TAS: Total antioxidant status; OSI=Oxidative stress index; n=Number of subjects; Significance p-value are denoted by*