Non communicable diseases, especially ASCVD contributes to world’s largest disease burden and a leading cause of mortality. India, being amidst this global ASCVD epidemic reports states that, between 2010 and 2013 out of 32% adult deaths, 23% were related to ASCVD [1]. Atherosclerosis is the cause of most cardiovascular events and atherosclerosis is accelerated by diabetes and the metabolic syndrome. Studies and statistics underline dysglycaemia as one of the most common non-communicable diseases in India and ASCVD being the most common cause of morbidity and mortality in such patients. Prediabetes, early in the diabetes mellitus spectrum comprises of Impaired Fasting Glucose (IFG) and Impaired Glucose Tolerance (IGT) and has an estimated Relative Risk (RR) for ASCVD of 0.97 to 1.30 for IGT and of 1.12 to 1.37 for IFG [2].
Since atherosclerosis initiates as arterial wall lesions and endothelial dysfunction, many studies have been done to assess the same. One such definitive indicator of atherosclerosis is an increase in CIMT of the arterial wall and CIMT correlates well with ASCVD risk factors such as blood pressure, lipid profiles, age and smoking as well as with the extent of coronary artery disease in both women and men. This has lead Food and Drug Administration (FDA) approve it as a valid technique to assess subclinical atherosclerosis [3,4]. The recent American College of Cardiology Foundation (ACC)-American Heart Association (AHA) guidelines suggest CIMT as a level IIa recommendation for cardiovascular risk evaluation (the same as for the ankle-brachial index and coronary-artery calcium scoring) in populations with Framhingham Risk Score (FRS) of 10-20% without known CAD, peripheral artery disease, cerebrovascular disease, or abdominal aortic disease [4].
CRP known to increase in inflammation and tissue injury within hours has been widely investigated in the context of atherosclerosis and vascular disorders since atherosclerosis and cardiovascular disease are considered to be due to chronic inflammatory processes. Based on multiple epidemiological and intervention studies, minor CRP elevation {high-sensitivity CRP (hsCRP)} has been shown to be associated with cardiovascular risk [5,6]. Elevation of hsCRP was found to be associated with risk of Type 2 DM in patients with metabolic syndrome and the levels correlated with the stage of beta-cell dysfunction and insulin resistance also [5].
The aim of this study was to measure CIMT and serum hsCRP levels as markers of subclinical atherosclerosis and chronic inflammation in prediabetics (defined by blood glucose levels, glycated haemoglobin (HbA1c), fasting insulin levels and insulin resistance) and compare them with normoglycaemic and also correlate them with HOMA-IR.
Materials and Methods
This case-control study was conducted in the Departments of Medicine, Biochemistry and Radiodiagnosis at Postgraduate Institute of Medical Education and Research, RML Hospital, New Delhi after getting clearance from Institutional Ethics Committee vide letter number TP(MD/MS)(74/2017)/IEC/PGIMER/RMLH 17/7/17 dated 30.11.2017.
Selection of Study Population
A total of 50 consecutive consenting prediabetic patients (as defined by fasting plasma glucose between 100 to 125 mg/dL OR 2 hour postprandial glucose/2 hour Oral Glucose Tolerance Test (OGTT) (after 75 gm of glucose solution ingestion) between 140 to 199 mg/dL OR HbA1c=5.7-6.4% (American Diabetes Association (ADA) 2016 guidelines) [7] either admitted in the In-patient Department (IPD) or visiting the Outpatient Department (OPD) of PGIMER, Dr. RML hospital between November 2017 and March 2019 were recruited. Fifty age and sex matched healthy controls were also taken from the consenting attendants of the patients admitted/taking OPD treatment from the hospital for any illness.
Exclusion Criteria
Known hypertensives, known diabetics, chronic smokers, chronic alcoholics, known cases of cerebrovascular accidents or Transient Ischaemic Attacks (TIA), known hypothyroid or hyperthyroid patients, known established cases of stroke, angina pectoris, myocardial infarction, known cases of peripheral vascular disease and history of intermittent claudication, known cases of Systemic Lupus Erythematosus (SLE), vasculitis, malignancy and connective tissue disorders, patients on drugs like statins and other anti-hyperlipidemic drugs and anti-platelet or anti-thrombotic drugs, patients with active inflammatory or infectious conditions were excluded from the study.
Calculation of Sample Size
The sample size was calculated with statistical inputs obtained from the study by Aydin Y et al., [8].
Aydin Y et al., suggests a mean CIMT of 0.81 (SD=0.2) in prediabetic patients and 0.64 (SD=0.16) in healthy volunteers.
Using these inputs, considering an alpha error of 5% with study powered up to 90% the required sample size was calculated to be 50 in each arm.
Clinical Examination
The study participants were called to the Department of Medicine, Dr. RML hospital and asked to fill a pre-determined questionnaire which included baseline data about age, sex, race, ethnicity and family history of diabetes or hypertension.
They then underwent a detailed clinical examination including measurement of height (using stadiometer), weight (using a weight measurement scale) and waist circumference at the upper borders of both hip bones (using a standard measuring tape). BMI was calculated as weight (kg) divided by the square of height (meters). Resting systolic and diastolic blood pressures were recorded twice using an automated sphygmomanometer after a 5-minute rest and average were calculated.
Laboratory Investigation
Around 10 mL of fasting blood sample was collected after venipuncture. Samples were also taken in EDTA vial for glycated haemoglobin measurements.
Investigation done on the patients were: plasma glucose (fasting and post prandial), HbA1c, fasting serum lipid profile, fasting serum insulin levels measured by Chemiluminescence Immunoassay (CLIA) on Vitros ECiQ by Orthoclinical Diagnostics.
All the samples were analysed on a fully automated clinical chemistry analyser in the Department of Biochemistry, PGIMER and Dr. RML Hospital, New Delhi.
Samples for hsCRP were centrifuged (3000 rpm for 10 minutes.) and stored in aliquots at -20°C in the Department of Biochemistry until batch analysed.
The basal state insulin resistance of the individual was calculated as HOMA-IR using the formula,
HOMA-IR=fasting insulin (μU/L)×fasting glucose (nmol/L)/22.5.
Measurement of hsCRP
hsCRP reagent was used on the VITROS 5,1 FS/4600 chemistry systems and the VITROS 5600/XT 7600 integrated systems to quantitatively measure hsCRP in the sample serum, initially stored at -20°C until batch analysed.
Principles of the Procedure
The VITROS chemistry products hsCRP reagent is a dual chambered package containing ready-to-use liquid reagents. Samples, calibrators and controls were mixed with reagent 1 containing a buffer. Addition of anti-CRP antibodies coupled to latex microparticles (Reagent 2) produced an immunochemical reaction yielding CRP antigen/antibody complexes. The turbidity was measured spectrophotometrically at 660 nm [Table/Fig-1]. Once a calibration had been performed for each reagent lot, the CRP concentration in each sample was determined using the stored calibration curve and the measured absorbance obtained in the assay of the sample.
Test type and conditions for immunochemical determination of hsCRP.
| Test type | VITROS system | Approximate incubation time | Temperature | Wavelength | Reaction sample volume |
|---|
| Two-point rate | 5600, XT 7600, 4600, 5, 1 FS | 8 minutes | 37°C (98.6°F) | 660 nm | 16 μL |
Test Type and Conditions
Interpretation of results was based on guidelines recommended by the US Centres for Disease Control and Prevention, and the American Heart Association for assessment of risk for cardiovascular disease in adults [9]. Classification for Cardiovascular Disease Risk Conventional and SI Units (mg/L) is as follows: low <1, average between 1-3, high between 3-10 and indeterminant >10.
Ultrasonographic Examination
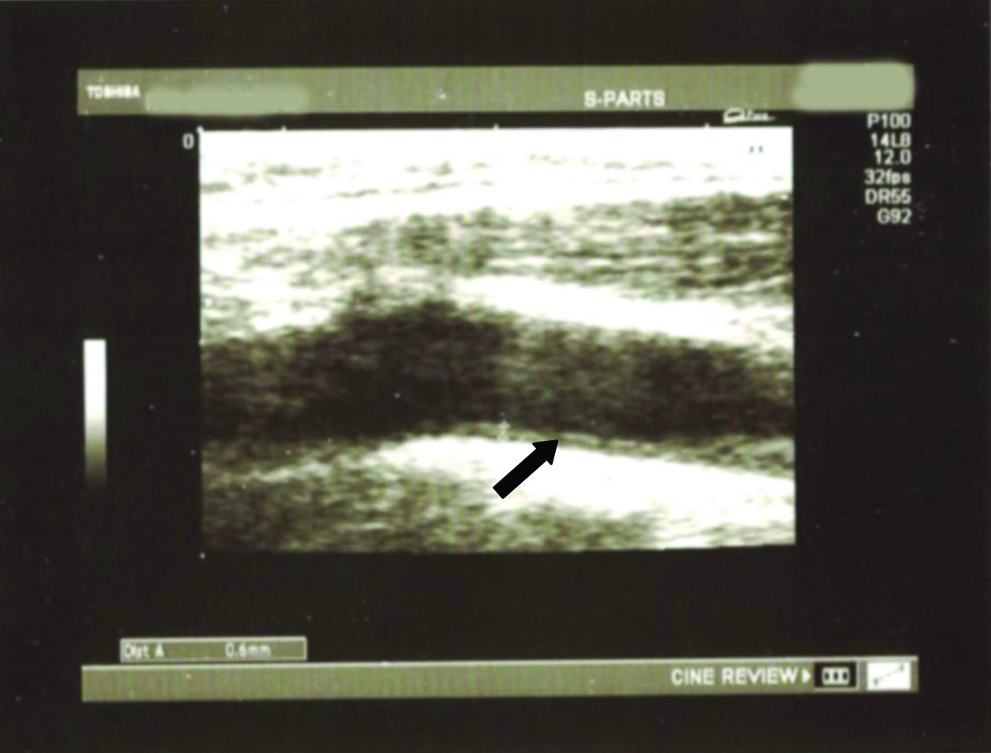
All cases and controls underwent high-resolution B-mode ultrasonography with a 7.5 MHz linear probe in the Department of Radiology, RMLH. CIMT was measured as the distance between the two echogenic lines (representing intima and media) as shown in the USG film from bilateral common carotids [Table/Fig-2] at a level proximal to the bifurcation of the Common Carotid Artery (CCA) and the mean was calculated and tabulated. All scans and image measurements were carried out by the same investigator, blinded to the status of the participants.
Arrow depicting intima media thickness. Picture showing the measurement of CIMT. The value recorded was 0.6 mm.
Statistical Analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean±SD and median. Normality of data were tested by Kolmogorov-Smirnov test. If the normality was rejected, then non-parametric test was used. Quantitative variables were compared using independent t-test/Mann-Whitney Test (when the data sets were not normally distributed) between the two groups. Qualitative variables were correlated using chi-square test/Fisher’s-Exact test. Spearman rank correlation coefficient was used to find out the correlation of various parameters with each other. Univariate linear regression was used to find out the cause and effect relationship between various parameters. A p-value of <0.05 was considered statistically significant. The data analysis was done using SPSS version 21.0.
Results
The mean age of cases and controls were 35.96±4.48 and 35.94±4.27 years respectively. In terms of age, sex distribution, waist circumference, systolic and diastolic BP cases and controls were matched [Table/Fig-3].
Demographic and anthropometric characteristics among cases and controls.
| Parameter | Cases (N=50) | Controls (N=50) | p-value |
|---|
| Age (Mean±SD) | 35.96±4.48 | 35.94±4.27 | 0.982 |
| Sex (%) |
| Males | 48 (n=24) | 54 (n=27) | 0.548 |
| Females | 52 (n=26) | 46 (n=23) | |
| BMI (Mean±SD) | 24.07±2.71 | 24.08±2.7 | 0.99 |
| Waist circumference (cm) (mean±SD) | 82.02±9.22 | 80.11±13.03 | 0.775 |
| Systolic blood pressure (mmHg) (mean±SD) | 116.56±17.08 | 114.8±9.00 | 0.521 |
| Diastolic blood presure (mmHg) (mean±SD) | 73.76±4.25 | 73.52±5.68 | 0.811 |
Fasting and postprandial blood sugar, HbA1C, Serum fasting insulin levels, HOMA-IR, triglycerides, LDL and HDL levels were significantly different between cases and control [Table/Fig-4]. However, there was no significant difference noted between the case and control in the levels of total and VLDL cholesterol [Table/Fig-4].
Biochemical parameters among cases and controls.
| No. | Biochemical parameters | Cases | Controls | p-value |
|---|
| 1. | Fasting blood sugar (mg/dL) | 110.86±9.79 | 86.68±7.14 | <0.0001 |
| 2. | Postprandial blood sugar (mg/dL) | 161.3±21.97 | 119.68±12.1 | <0.0001 |
| 3. | HbA1c | 6±0.21 | 4.9±0.46 | <0.0001 |
| 4. | Fasting insulin levels (mIU/L) | 12.22±5.42 | 5.37±1.95 | <0.0001 |
| 5 | HOMA-IR index | 3.31±1.56 | 1.16±0.44 | <0.0001 |
| 7. | Triglycerides (mg/dL) | 123.4±32.26 | 103.32±27.79 | 0.0005 |
| 8. | LDL-cholesterol (mg/dL) | 104.08±20.72 | 97.41±16.29 | 0.047 |
| 9. | VLDL-cholesterol (mg/dL) | 25.1±7.32 | 24.68±7.56 | 0.778 |
| 10. | Total cholesterol (mg/dL) | 143.02±28.25 | 132.24±38.4 | 0.113 |
| 11. | HDL-cholesterol (mg/dL) | 38.86±9.2 | 49.02±7.09 | <0.0001 |
| 12. | Serum hsCRP (mg/L) | 5.75±4.16 | 1.86±1.67 | <0.0001 |
| 13. | Mean CIMT (in mm) | 0.59±0.11 | 0.45±0.07 | <0.0001 |
HOMA-IR index showed mean values of 3.31±1.56 and 1.16±0.44 in cases and controls respectively [Table/Fig-5] and the difference, statistically significant (p<0.001). The mean serum fasting insulin level among cases was 12.22 mIU/mL and that of the control group was 5.37 mIU/mL and the p-value was <0.0001 [Table/Fig-6].
HOMA-IR index among cases and controls.
| HOMA-IR | Cases | Controls | p-value |
|---|
| Sample size | 50 | 50 | ≤0.0001 |
| Mean±SD | 3.31±1.56 | 1.16±0.44 |
| Median | 3.3 | 1.1 |
| Min-Max | 0.1-6.1 | 0.4-2.5 |
| Inter quartile range | 2.300-4.600 | 0.800-1.500 |
Serum fasting insulin levels.
| Serum fasting insulin levels | Cases | Controls | p-value |
|---|
| Sample size | 50 | 50 | ≤0.0001 |
| Mean±SD (mIU/L) | 12.22±5.42 | 5.37±1.95 |
| Median (mIU/L) | 11.9 | 5.45 |
| Min-Max (mIU/L) | 2.12-23.8 | 2.13-10.8 |
| Inter quartile range | 7.800-16.800 | 3.800-6.800 |
The mean values of hsCRP [Table/Fig-7,8] in cases and controls were 5.75±4.16 mg/L and 1.86±1.67 mg/L respectively with a statistically significant difference (p<0.0001). The graph depicts that 48% (almost half) cases and 12% of controls fell in the high-risk category. However, 28% cases and 52% controls fell in the average risk category. Only 4% of cases but 36% controls were in the low risk category.
Serum hsCRP levels in cases and controls.
| Serum hsCRP | Cases | Controls | p-value |
|---|
| Sample size | 50 | 50 | ≤0.0001 |
| Mean±SD (mg/L) | 5.75±4.16 | 1.86±1.67 |
| Median (mg/L) | 3.63 | 1.35 |
| Min-Max (mg/L) | 0.57-15 | 0.31-6.38 |
hsCRP range among cases and controls-risk stratification of cardiovascular disease.
| Variables | Group | Total | p-value |
|---|
| Case | Control |
|---|
| Serum hsCRP (mg/L) | 1) <1 (Low) | 2 (4.00%) | 18 (36.00%) | 20 (20.00%) | <0.0001 |
| 2) 1-<3 (Average) | 14 (28.00%) | 26 (52.00%) | 40 (40.00%) |
| 3) 3-10 (High) | 24 (48.00%) | 6 (12.00%) | 30 (30.00%) |
| 4) >10 (Indeterminant) | 10 (20.00%) | 0 (0.00%) | 10 (10.00%) |
| Total | 50 (100.00%) | 50 (100.00%) | - |
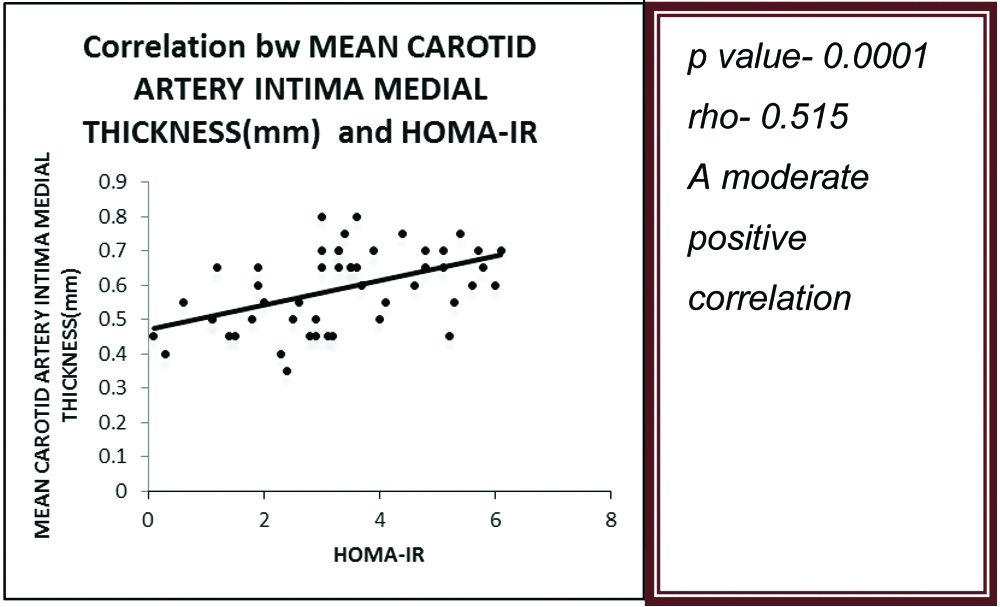
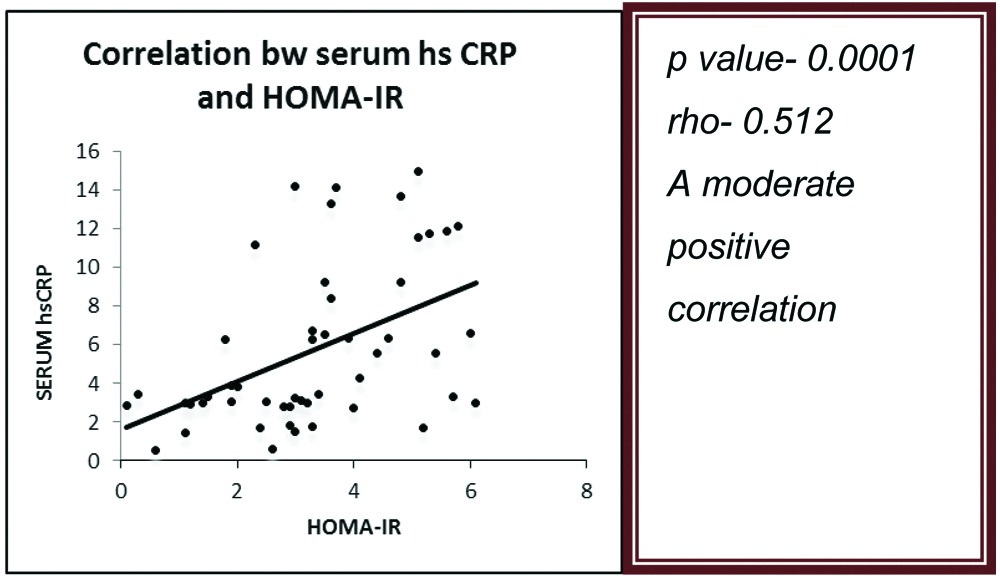
The mean value of CIMT in cases was 0.59±0.11 mm and that in controls was 0.45±0.07 mm (p<0.0001) [Table/Fig-9]. There was a moderate positive correlation between mean CIMT and HOMA-IR in the prediabetic subjects (p-value=0.0001) (ρ=0.515) [Table/Fig-10]. There was also a moderate positive correlation between serum hsCRP levels and HOMA-IR in the prediabetic subjects (p-value=0.0001) (ρ=0.512) [Table/Fig-11].
Mean Carotid Artery Intima Media Thickness (CIMT) among cases and controls.
| Mean carotid artery intima media thickness (mm) | Cases | Controls | p-value |
|---|
| Sample size | 50 | 50 | ≤0.0001 |
| Mean±SD | 0.59±0.11 | 0.45±0.07 |
| Median | 0.6 | 0.45 |
| Min-Max | 0.35-0.8 | 0.3-0.6 |
| Inter quartile range | 0.500-0.700 | -0.500 |
Correlation of HOMA-IR with CIMT.
Correlation of HOMA-IR with hsCRP.
Linear regression analysis showed a 1.256 unit increase in hsCRP for a unit increase in HOMA-IR [Table/Fig-12]. Linear regression analysis also showed a 0.035 unit increase in CIMT for a unit increase in HOMA-IR [Table/Fig-13].
Linear regression analysis of HOMA-IR and hsCRP.
| Serum hsCRP(mg/L) | Unstandardized coefficients | p-value | 95.0% Confidence interval for B |
|---|
| B | Std. error | Lower bound | Upper bound |
|---|
| HOMA-IR | 1.256 | 0.340 | 0.001 | 0.573 | 1.939 |
For increase in one unit of HOMA IR, serum hsCRP increases by 1.256
Linear regression analysis of HOMA-IR and CIMT.
| Mean CIMT (mm) | Unstandardized Coefficients | p-value | 95.0% Confidence Interval for B |
|---|
| B | Std. Error | Lower Bound | Upper Bound |
|---|
| HOMA-IR | 0.035 | 0.009 | 0.0004 | 0.017 | 0.054 |
For increase in one unit of HOMA IR, CIMT increases by 0.035
The hsCRP values were also categorised as per the standard reference values of <1, 1-3, 3-10, >10 [Table/Fig-8]. It was found that 4% cases and 36% of controls had values <1 and thus, a low risk of ASCVD; 28% cases and 52% controls had a value of 1-3, with an average risk; 48% cases (around half of the subjects) and 12% controls had a value of >3-10 falling in the high risk group and rest being indeterminate. This allows us a mean difference between the two groups being statistically significant (p-value <0.0001) and the data suggests that the predominant percentage of cases fall in the high-risk category further strengthening the association between prediabetes and ASCVD.
Discussion
The study showed that serum hsCRP levels and CIMT values are increased in pre-diabetics when compared to normoglycaemic. It also showed a positive correlation of the variables with HOMA-IR. This indicates that there is a definitive atherosclerotic and cardiovascular risk associated with the prediabetic state and that insulin resistance might underlie the same.
The development of microvascular and macrovascular complications of diabetes mellitus have been observed to start well before the onset of clinical disease and this has been explained by the underlying insulin resistance forming the core of the inflammatory process [10,11]. It has been proposed that glucose might affect cells of the artery wall, including endothelial cells, smooth muscle cells, and macrophages, directly or indirectly via the generation of Advanced Glycation End-products (AGEs) or reactive oxygen species [12].
Treating insulin-resistant patients with statins (LDL-lowering agents) or Angiotensin-converting Enzyme (ACE) inhibitors (blood pressure-lowering agents) has been found to decrease atherosclerotic complications. These benefits accrue even though LDL cholesterol levels are not usually elevated in insulin resistance and hypertension is less closely associated with insulin resistance than are other metabolic abnormalities [13].
The Insulin Resistance Atherosclerosis Study found that insulin resistance was significantly related to higher levels of CRP, fibrinogen, and Plasminogen activator inhibitor-1 (PAI-1) in serum and that high PAI-1 and CRP levels predicted the development of diabetes [14]. Present study shows that about 39 patients (78%) of prediabetics had HOMA-IR values of 2 or above, indicating a significant insulin resistant state, the means being 3.31±1.56 and 1.16±0.44 in cases and controls respectively.
Studies show that prediabetes was associated with an increased risk of all-cause mortality, composite cardiovascular events, coronary heart disease, stroke when it was defined with IFG-ADA, IFG-WHO, impaired glucose tolerance, HbA1c [15].
Sabanayagam C et al., analysed data from two Singapore based studies and noticed that elevated high sensitivity CRP (hsCRP) levels were found to be associated with prediabetes. The OR of prediabetes in persons with hsCRP 1-3 mg/L and >3 mg/L was 1.31 and 2.17 respectively [16]. Our study shows a significant proportion of prediabetics falling under the average and high-risk categories based on hsCRP (28% cases with a value of 1-3 and an average risk and 48% cases (around half of the subjects) with values 3-10 and a high risk for ASCVD.
A cross-sectional study by Adolphe A and Huang CX, showed that Intima-media thickness increased with every additional component of the MetS, from 0.516 mm for 0 components to 0.688 mm for 4 or more components (p<.001) [17]. Faeh D et al., studied the relation between IGR and IMT. Age-adjusted levels of the major ASCVD risk factors and IMT worsened gradually across IGR categories (NFG<IFG/NGT<IFG/IGT<DM) and they proposed that IGR patients should be periodically screened and managed [18]. The mean value of CIMT in cases in the present study was 0.59±0.11 mm, most of the values exceeding the 75th percentile for age specific values of CIMT [19].
Parildar H et al., aimed to assess CIMT and serum high-sensitivity C-reactive protein (hs-CRP) levels in prediabetic patients. Serum hs-CRP levels, left, right and maximum CIMT were statistically higher among prediabetics compared to control group. There was also a positive, significant correlation among CIMT and fasting blood glucose, HbA1c, hs-CRP levels and BMI [20]. A cross-sectional, case-control study done in Indian Obese Children (6-17 years) and Adolescents by Dabas A et al., and team showed that CIMT correlated significantly to blood pressure, insulin sensitivity (HOMA and MATSUDA indices), and body fat (BMI and Fat mass index) [21].
The present study showed increased values of hsCRP, CIMT in prediabetics. Moreover, it also shows a positive correlation of the atherogenic indices with HOMA-IR used as a measure of insulin resistance, which signifies the role of insulin resistance in contributing to the ASCVD risk in these patients.
Thus, the index study is one of the very few Indian studies researching the probability of a prediabetic heart disease and atherosclerosis. The strength of the study is that it assesses the correlation of the atherogenic parameters with HOMA-IR, in contrast to the other studies on prediabetic ASCVD employing blood sugar levels and HbA1c.
An estimated 30.3 million people of all ages or 9.4% of the U.S. population-had diabetes in 2015 [22]. An ICMR-INDIAB population-based cross-sectional study by Anjana RM et al., showed that in 2017, the overall prevalence of diabetes and prediabetes in all 15 states of India studied were 7.3% (95% CI 7.0-7.5) and 10.3% (10.0-10.6) [23]. Given the high prevalence of the diabetes spectrum disorders in a country like ours and their deleterious effects on health, strategies to devise early diagnosis and management of both the disease and the complications are the need of the hour. Clinicians shouldn’t stop with diagnosing prediabetics but also have to go an extra mile in detecting the complications at an earlier stage and cater to the high-risk candidates. This would pave way to primary prevention of diabetes and ASCVD related morbidity and mortality in these patients.
The ADA recommends an intensive diet and physical activity program for all patients with prediabetes and metformin in some cases [24]. Schwarz PE et al., reported that in patients with impaired glucose tolerance, a change in daily ambulatory activity can significantly decrease the risk of cardiovascular events [25]. Studies have also demonstrated a protective and a preventive effect with statins, metformin, rosiglitazone and other insulin sensitising agents in patients with prediabetes, prehypertension and metabolic syndrome and a decrease in CRP levels following treatment [26-28]. However, such trials are limited and require further population-based research.
Limitation
This study was conducted only in a subset of the urban Indian population (already prone to the metabolic syndrome spectrum due to influences of diet and lifestyle changes) who belong to a geographic area. More studies are needed in a wider and mixed population to establish a relationship between the variables bringing ethnicity, geographical distribution into account and find how they affect individuals, the values of hsCRP and CIMT.
Conclusion
Insulin resistance is a major contributor to atherosclerosis and ASCVD and CIMT and hsCRP are invaluable markers of subclinical atherosclerosis to diagnose them in prediabetic patients. The present authors propose early assessment of prediabetics for evidence of ASCVD and take necessary action early in the course. Further studies in a wider population and with pharmacological trials are essential to offer significant proof of benefit to the at-risk population.
For increase in one unit of HOMA IR, serum hsCRP increases by 1.256
For increase in one unit of HOMA IR, CIMT increases by 0.035