Post-Treatment Endodontic Disease (PTED) is a persistent infection of the pulp and periapical tissue that occurs after the root canal has been treated and obturated. Epidemiological research showed that the occurrence of PTED is high [1]. The main cause of PTED is microorganisms that are able to survive against the root canal treatment along with the antimicrobials used. The most common microorganism found in the case of persistent infections is Enterococcus faecalis [2]. The amount of E. faecalis found in persistent infections was reported to be nine times more than the amount of E. faecalis found in the cases of primary endodontic infection [3]. To date, no adequate information is provided to explain how these bacteria are able to survive [4].
Endodontic treatment aims to eliminate any microorganisms along with their metabolism products; this also includes E. faecalis bacteria. The use of irrigation solutions and medicaments in the root canal are done in order to help eliminate any microorganisms that cannot be removed through mechanical preparation. The commonly used irrigation material is sodium hypochlorite (NaOCl) with a concentration between 0.5-5.25%. This solution can penetrate into the dentinal tubules properly and able to dissolve any organic substances. However, as an irrigation material, it has a pungent smell, unable to dissolve inorganic substances, and toxic; higher concentration may provide a stronger antibacterial effect, but so does its toxicity [9]. The effective concentration of NaOCl reported against E. faecalis is 5%. Chlorhexidine is an alternative irrigation material to NaOCl because it is relatively non-toxic and has a broad-spectrum antimicrobial properties, along with its relatively low side effects [10].
Chlorhexidine can be found in the form of a gel or liquid. Several concentrations of CHX normally used today are 0.1%, 0.2%, and 2%. Chlorhexidine with a concentration of 2% can be used as an alternative irrigation material; it is considered that a 2% CHX has the same ability as a 5.25% NaOCl solution without any irritation side effect. Chlorhexidine has a bacteriostatic property at a concentration of 0.2% and bactericidal properties at a concentration of 2%, it will be then absorbed into the dental tissues and mucous membranes and released gradually at a therapeutic level. In an in-vitro research, it was revealed that the time needed by CHX 1% and 2% to eliminate all microorganisms was the same as the time needed by NaOCl 5.25% [10].
The aim of this research was to evaluate the antimicrobial efficacy of various concentrations of CHX against E. faecalis bacteria in the endodontically treated root canals.
Materials and Methods
The present in-vitro study was done using E. faecalis isolates from endodontic patients, in Department of Conservative Dentistry in collaboration with Department of Oral Biology in University of Indonesia, Jakarta from January 2013 to September 2013. The study was done after taking the Institutional Ethical committee clearance (94/Ethical Clearance/FKGUI/I/2012). The number of subjects in this study were 30 patients (7 subjects with persistent cases, 17 subjects with pulp necrosis cases, and 6 subjects with abscess cases).
Informed consent was obtained from all the participants involved in the study.
The inclusion criteria for the subjects applied in this research are:
Male and female subjects aged 15-55 years with a diagnosis of infection in endodontically treated tooth/reinfection of root canal (persistent case).
Cases of primary endodontic infection including pulp necrosis, or periapical abscess.
All permanent molar teeth should have erupted completely; and with good general condition (healthy physical condition without any systemic abnormalities).
The exclusion criteria are:
Those who did not adhere to the research.
Those who did not undergo a complete treatment.
Those who refused to sign an informed consent.
The research began with an isolation of E. faecalis samples from patients’ teeth that have been endodontically treated, continued by culturing and followed by exposure of E. faecalis to CHX with a concentration of 0.1%, 0.2%, and 2%, respectively, with an incubation period of half, one, and three hours. The calculation of viability of E. faecalis was done with MTT assay.
Sampling Technique for Pre and Post Groups
Samples were taken only from patients whose treatment was just about to begin and patients with repeated treatment (post-treatment) and those who currently underwent the treatment were excluded. The oral cavity of patients was first isolated with rubber dam and cotton rolls. All tooth restorations and caries defects were excavated. After access preparation was done, the root canal was irrigated with a sterile saline solution in order to remove any remaining obturation material and the root canal were lubricated before the collection of the sample were done. The root canal was prepared with no. 15 sterile K- file adjusted for the working length. Then, three sterile paper points were inserted into the root canal for 60 seconds and then moved into a 1.5 mL sterile micro tube containing 500 μL Phosphate Buffered-Saline (PBS) liquid.
Culturing the E. faecalis Samples
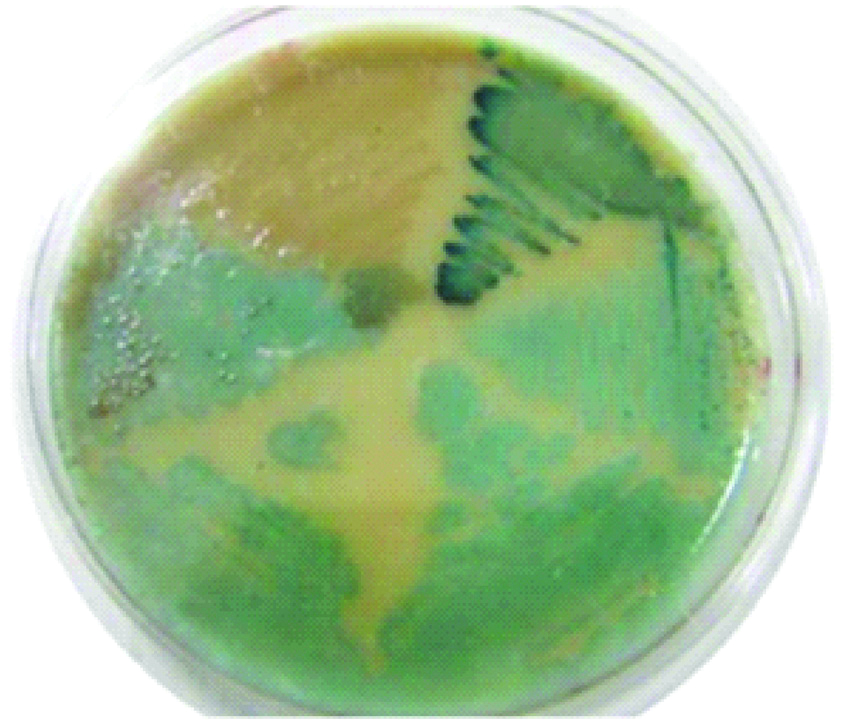
To ensure the survival of E. faecalis, the samples in the micro tube were homogenised by turning it over, taken as much 10 μL, and spreaded over a petridish containing CHROMagarTM and flattened with a burnisher. The petridish was closed and then put into an anaerobic jar which had been filled with gas packs (AnaerobGen compact). After being incubated for 24 hours at 37°C, the E. faecalis would appear bluish green.
Identification of E. faecalis colony: The viability of the colonies believed to contain E. faecalis colony was proven by MTT assay (4.5- Dimethylthiazol 2-yl), (2.5- diphenyltetrazolium bromide assay).
This step was first preceded by DNA extraction using the Genomic Extraction Kit Gene Jet Fast used to identify E. faecalis. The DNA was used as a template for Polymerase Chain Reaction (PCR) which consisted of 12.5 μL GeneJet Fast PCR Master Mix, 3 μL forward primer, 3 μL Reverse primer, 3.5 μL Water nucleus free, so making it 22 μL for the total volume. The primers used were reversed (Rv) GGG GAC GTT CAG TTA ACG TT, and Forward (Fw) TGG CAT AAG AGT GAA AGG CGC at 290 bp. The PCR amplicons were visualised using electrophoresis technique by first making an agarose with a concentration of 1.5%. In this study, 50 mL of tray was used so the total agarose powder (Fermentas®) used was 50 mL×1.5%=0.75 g. After it was mixed with Tris/Borate/EDTA (TBE) Buffer (IX: Tris 107.78 g/L, EDTA-N2-salt 7.44 g/L, boric acid 55 g/L) and reaching a volume of 50 mL, the mixture was then put in the microwave for one minute and then moved into the combed tray after being cooled for about three minutes on the shaker machine. The mixtures were left as such until it hardened perfectly. The process of inserting PCR results into the well was done by mixing the loading dye (Fermentas®) as much as 1/6th of the total volume. Then, the electrophoresis process was carried out using the TBE Buffer delivery fluid (IX: Tris 107.78 g/L, EDTA- N2-salt 7.44 g/L, boric acid 55 g/L) with the default setting of 80 volts, 400 mA, 75 minutes (if using one comb) or 55 minutes (if using two combs). The DNA ladder (GeneRuler 100 bp, Fermentas®) used was 2 μL. After the process was finished, the gel was inserted into the documentation gel machine after it was soaked in ethidium bromide liquid for 10 minutes and rinsed for 30 minutes under the running water. The PCR results could be documented on printed paper or digitally as computer files.
Viability of E. faecalis with MTT Assay
The next step was to test the viability of E. faecalis after being exposed to CHX solution. The test used the MTT assay (4.5- Dimethylthiazol-2-yl), (2.5-diphenyltetra-zolium bromide assay). E. faecalis was exposed to CHX with a concentration of 0.1%, 0.2%, and 2%, respectively, with an incubation period of half, one, and three hours.
The cells were then suspended into a colony of 106 cells/mL, and reincubated overnight. On the next day, one well was exposed to Enterococcus faecalis; this sample was then put into 96 wells with each well having a volume of 50 μL. There was one column of wells that was not exposed to bacteria in order to serve as a control group. After this, the cells were reincubated for four hours.
After four hours of incubation, the supernatant formed was removed and the wells were rinsed with PBS solution once. The MTT solution was then put into each well and they were incubated again for three hours, after which 150 μL of acidified isopropanol 0.04 N were added into each well, and finally 96 micro well plates were placed in the orbital shaker at 50 rpm for one hour. This process was repeated twice.
The MTT assay result is interpreted with a micro plate reader with a wavelength of 490 nm in order to obtain an absorbance/optical density (OD) value. The cell viability value was calculated using the following formula.
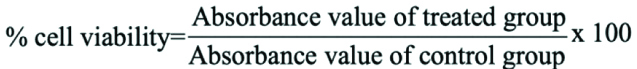
Statistical Analysis
The data was analysed by comparing Mean±SD of the two groups i.e., pre and post endodontically treated teeth. For this, Anova test was applied. The p values was set at p<0.05 to be statistically significant.
Results
The effect of various concentrations of CHX against the viability of E. faecalis isolates both in the pre-treatment and post-treatment group based on the MTT test results and also the relationship between the viability and exposure time are shown in [Table/Fig-1a,b].
The viability (%) of Enterococcus faecalis colony in a medium containing various concentrations of chlorhexidine-Pre endodontic treatment group.
| Viability (%) |
|---|
| Sample source | Half hour | One hour | Three hour |
| Average±SD | Average±SD | Average±SD |
| Pre endodontic treatment: Root Canal (n=23) | |
| Chlorhexidine control | 100 | 100 | 100 |
| 0.1% Chlorhexidine | 69.96±7.32 | 43.14±6.79 | 60.01±7.69 |
| 0.2% Chlorhexidine | 71.49±6.47 | 41.31±6.51 | 60.42±7.40 |
| 2% | 76.20±5.94 | 47.94±6.77 | 65.43±7.21 |
The viability (%) of Enterococcus faecalis colony in a medium containing various concentrations of chlorhexidine- Post endodontic treatment group.
| Viability (%) |
|---|
| Sample source | Half hour | One hour | Three hour |
| Average±SD | Average±SD | Average±SD |
| Post endodontic treatment: Root canal (n=7) |
| Chlorhexidine control | 100 | 100 | 100 |
| 0.1% Chlorhexidine | 36.98±12.38 | 48.86±17.27 | 51.29±11.44 |
| 0.2% Chlorhexidine | 39.86±11.93 | 49.23±15.97 | 55.72±11.18 |
| 2% | 43.68±12.93 | 52.33±16.08 | 61.94±12.48 |
In the pre-treatment patients group, considering that the analysis results were based on the impact of incubation time, between three concentrations of CHX, it is shown in the [Table/Fig-1a] that from half hour to one hour of incubation time, CHX effectiveness increased, although this trend was decreased after three hours of incubation time. Based on effectiveness of CHX, when compared to the control group, the three concentrations can decrease the viability of E. faecalis significantly (p<0.05). The viability of E. faecalis colony after being exposed by CHX 0.1%, 0.2%, and 2% were specifically different (p>0.5%) [Table/Fig-1b] The growth of E. faecalis on chrom agar medium showed bluish-green colonies as can be seen in [Table/Fig-2].
Growth of E. faecalis on chrom agar medium.
In the patients’ endodontically treated root canals, when analysed based on the impact of incubation time, it was seen from the table that as the CHX incubation time increase, it will also cause an increase in the average number of E. faecalis colony, regardless of the concentrations of CHX. It indicated that the exposure of CHX 0.1%, 0.2%, and 2% to the E. faecalis colony from the subjects’ root canal both before and after the endodontic treatment will increase the average number of E. faecalis colony if it was incubated for half, one, or three hours.
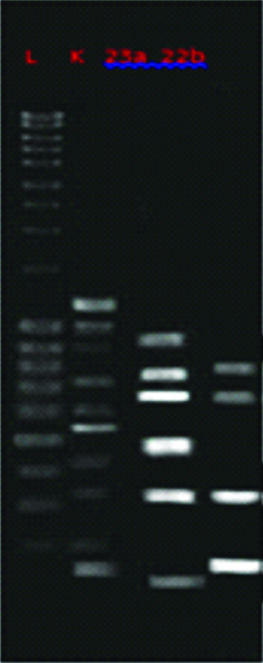
Genotype profiling of E. faecalis used in the study is shown in [Table/Fig-3] showed.
Differences in genotype profiling of E. faecalis L= ladder, K=control (E. faecalis ATCC 2912).
Discussion
E. faecalis had been known widely as a strain that could survive in the endodontically treated root canals [11-13]. The frequency of E. faecalis existence in the case of post endodontic treatment, persistent infection was found to be nine times larger than the primary endodontic infection [14,15].
The anatomical complexity of the root canal paired with the limited access of instruments used in the treatment often became the obstacles for the success of endodontic treatment [16]. The use of irrigation solution such as sodium hypochlorite (NaOCl) with the concentration of 0.5-5.25% [17] or CHX with the concentration of 0.1-2% had been generally approved to be able to reduce and remove microorganisms from root canal, however the optimal concentration and duration of exposure of the irrigation solution in order to increase the effectiveness of killing bacteria in the root canal is still needed to be known further. This study evaluated the antimicrobial efficacy of various concentrations of CHX against E. faecalis bacteria in the endodontically treated root canals with an incubation time of thirty minutes, one hour, and three hours based on the finding that during time period, E. faecalis was still in exponential growth phase (active fission), thus the effectiveness of the irrigation solution during the time periods also needed to be observed.
The effect of the incubation time and concentration of CHX to the viability of E. faecalis colony based on the MTT assay.
Chlorhexidine killed bacteria through the mechanism in which the cell wall of bacteria was damaged by the material. Based on the result of colony measurement, it was demonstrated that the use CHX with a concentration of 0.1%, 0.2% and 2% during half hour, one hour, and three hours showed a significant decrease in the colony viability of E. faecalis (p<0.05), however observation results based on MTT assay also showed that the increase in concentration was not associated with the bigger decrease in viability. Wang QQ et al., in their study investigated the prevalence of E. faecalis in saliva and filled root canals of teeth associated with apical periodontitis. They observed greater prevalence of the bacteria in root canals than in saliva, approximating 38% and 19% respectively. Inadequate and unsatisfactory obturation resulted in more bacterial species in root canals [18]. Vidana R et al., aimed to determine the origin of E. faecalis infection in root canals. Samples were taken from root canals, saliva and faeces and were subjected to microbiological culturing. The genetic analysis revealed bacteria from root canals were unrelated to normal gastrointestinal microflora thus, confirming their exogenous origin [19]. Pires-Bouças et al., observed that gelatinase is an impending virulence element in enterococcus. Its activity is prejudiced by numerous factors such as temperature, pH, divalent cations etc [20]. Therefore, CHX 0.1% tend to decrease the E. faecalis viability more when compared with CHX 0.2% in the present study.
Incubation time also played a role in determining the effectiveness of irrigation materials against the bacteria. Based on observation, colony number, and MTT viability, generally each incubation time periods of half hour, one hour, and three hours did not show any significant differences. However, when the incubation time periods in MTT assay (half, one, and three hours) were compared to each other for the E. faecalis viability isolated from the patients’ pre-treated root canal, the results showed a significant difference between half hour and one hour. Based on this result, it can be determined that incubation time between half hour and one hour cause a significant decrease in E. faecalis viability. As the material exposure time to the bacteria increase, it would maximise the action of CHX to destroy the cell wall of the bacteria. However, at a certain particular time period, the effectiveness of irrigation materials will not give any significant bacteria decrease due to the decreasing efficacy of the antimicrobial agent.
Kim HS et al., demonstrated comparable antibacterial effect of 1% Alexidine and 2% CHX against E. faecalis proposing that both can be used as final rinse after intracanal medication or as canal soaking agents. Ruptured or antiseptic attached bacteria were more in 10 minutes soaked group as compared to the five minutes soaked group [21].
Nair PN et al., assessed the in-vivo intracanal microbial status of apical root canals of molars with apical periodontitis. They concluded that complexity in anatomical configuration and organisation of microflora in the unapproachable root canals validate the need of rigorous application of chemo-mechanical measures in necrotic canals. This is needed to reduce the biofilm and exceed the favourable prognosis of treatment [22].
E. faecalis in the post-treatment group tended to be more resistant to the exposure of CHX irrigation materials, thus they tend to be able to survive against this material. Koljalg S et al., (2002) stated that E. faecalis is a gram positive bacteria with the highest resistance against CHX compared to the other gram positive bacteria such as Staphylococcus aureus [23]. The differences between the pre-treated and post-treated root canal condition may influence the bacterial growth and existence. Nutritional limitation and antimicrobial activity may be able to decrease or even completely kill sensitive strain, but for persistent species like E. faecalis, they may still able to survive [24]. Furthermore, E. faecalis has the ability to survive in an environment which lack in nutrition by decreasing its metabolism level [15].
Savitha A et al., concluded that 2% CHX with chitosan group yielded highest microbial reduction against E. faecalis during retreatment of failed endodontic cases [25].
Ferraz CCR et al., compared the antimicrobial efficacy of CHX gel, CHX solution and NaOCl as endodontic irrigants. The results indicated that gel has greater effect as endodontic chemical substance [26].
Limitation
The study had certain drawbacks owing to its small sample size. Though three different concentrations were analysed but the comparable alternative irrigant was lacking in the study. Further studies are recommended to investigate the different groups of irrigants with larger sample size and at different concentrations.
Conclusion
Chlorhexidine with concentrations of 0.1%, 0.2%, and 2% are generally effective against E. faecalis where CHX 0.1% showed the highest decrease in the viability of E. faecalis; an incubation time of 30 minutes has been shown in this study to be significantly effective in terms of decreasing the E. faecalis viability.