A clinical condition of the distended abdomen due to excess accumulation of fluid in the abdominal cavity is called ascites [1,2]. Runyon BA and colleagues reported that 80% of ascites is caused by parenchymal liver disease and then malignancy 10%, heart failure 5%, tuberculosis 2% and other causes in 3% of cases [3]. Among malignant ascites, ovarian cancers are the most common primary tumours and sixth most common malignant neoplasm in women with the highest mortality rate [4]. Malignant ascites can be confirmed with the cytological presence of tumour cells in the ascitic fluid. Runyon BA et al., reported that approximately 97% of patients with peritoneal carcinomatosis had positive ascitic fluid cytology [5]. Lymphatic resorption gets decreased by the increased vascular permeability which causes gross changes in the concentrations of functional proteins and metabolites resulting into ascites. The aggressive clinical presentation of the abdomen, differential diagnosis, and management of these patients poses a challenging task. Thorough clinical assessment and optimal utilisation of imageology and laboratory tests enable to clinch the diagnosis of ascites and understand the underlying mechanisms [6].
Many studies were concentrated on the analysis of ascitic fluid to solve the problem of differential diagnosis and discover some reliable cytological and biochemical markers [7-10]. Pare P et al., found Serum Ascitic Albumin Gradient (SAAG) better for discrimination of portal hypertension than ascitic fluid protein concentration [11]. SAAG is considered a useful clinical tool for diagnosis of ascites [12]. SAAG is generally high (≥1.1 g/dL) in portal hypertension related ascites (liver cirrhosis or congestive heart failure [12-15] and low (<1.1 g/dL) in ascites not due to portal hypertension as in cases of infection or malignancy. The accuracy of the SAAG is approximately 97% in classifying ascites related to portal hypertension whereas only 55% was identified using ascitic total protein concentration [10]. British and American guidelines have adopted SAAG as an initial testing strategy for the differential diagnosis of ascites [16].
The conventional tests such as SAAG, fibronectin, Carcino Embryonic Antigen (CEA), Cancer Antigen 125 (CA125), polymorph neutrophilic count, and microbial identifications were used for the differential diagnosis. However, heterogeneity in their aetiology and their clinical complications are a major concern. Further, it is mandatory to explore precise markers in the early and differential diagnosis, for better monitoring and their prevention in ascitic patients. Hence, the aim of the study was to estimate the levels of cholesterol, Serum Ascitic Triglycerides Gradient (SATG) and Serum Ascitic Cholesterol Gradient (SACG) along with SAAG and to differentiate ovarian cancer from liver cirrhosis ascitic patients.
Materials and Methods
The present prospective cross-sectional study was carried out between December 2016 and June 2019 in the Department of Gastroenterology, Osmania General Hospital, Hyderabad, Telangana, India. The patient’s history was recorded in the specific proforma, oral and written consent statement was obtained from all the patients who were enrolled in this study and the study was approved by the Ethics Scientific committee of Osmania Medical College/Hospital, Hyderabad.
A total of 375, histopathological, radiological and cytologically confirmed cases of liver cirrhosis and ovarian cancer ascitic patients without the involvement of renal, Pancreatic and tuberculosis were included in this study. On the same group of patients the authors performed a study taking into account the oxidative parameters [17].
Liver cirrhosis ascitic patients (n=230) venous blood and ascitic fluid samples were collected from Osmania General hospital, whereas ovarian cancer ascitic patients (n=145) samples were collected from MNJ Institute of Oncology and Regional Cancer Centre and Basavatarakam Indo-American Cancer Hospital and Research Institute, Hyderabad. A total of 150 blood samples (Controls) of age and sex-matched were collected from healthy volunteers, screened randomly and recruited in this study (Hospital working staff and patient’s attendant). Serum was separated and the investigations were done on the same day of collection, remaining specimen stored at -70°C.
Biochemical parameter like serum and ascitic fluid Total Proteins (TP) and Albumin (ALB) was estimated by Biuret and Bromocresol Green (BCG) End-point colorimetric method, Cholesterol (CHOL) and Triglycerides (TG) by (CHOD-PAP; GPO-PAP) Enzymatic colorimetric determinations, respectively using commercial kits from Erba Mannheim Transasia Bio-medicals Ltd. SAAG, SACG and SATG were determined by calculation method, subtracting ascitic fluid albumin, cholesterol and triglycerides concentrations from their respective serum concentrations. Calibrators, normal and abnormal controls were obtained from Randox laboratories and used to run for every batch for quality control and standardisation purpose.
Statistical Analysis
The statistical significance was determined by one-way ANOVA; SPSS (version-22) followed by post-hoc test by LSD (least significance difference) for significance. A p-value <0.05 was considered statistically significant.
Results
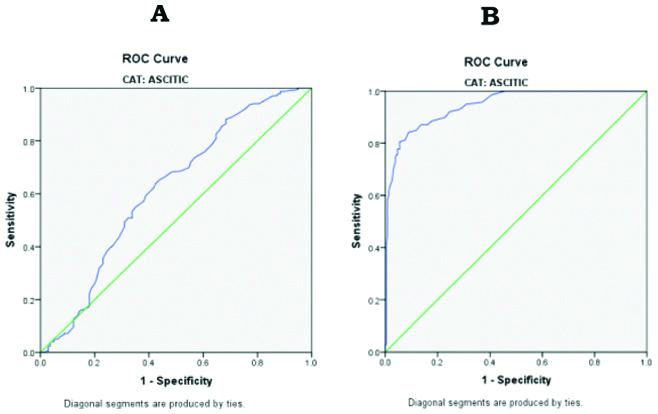
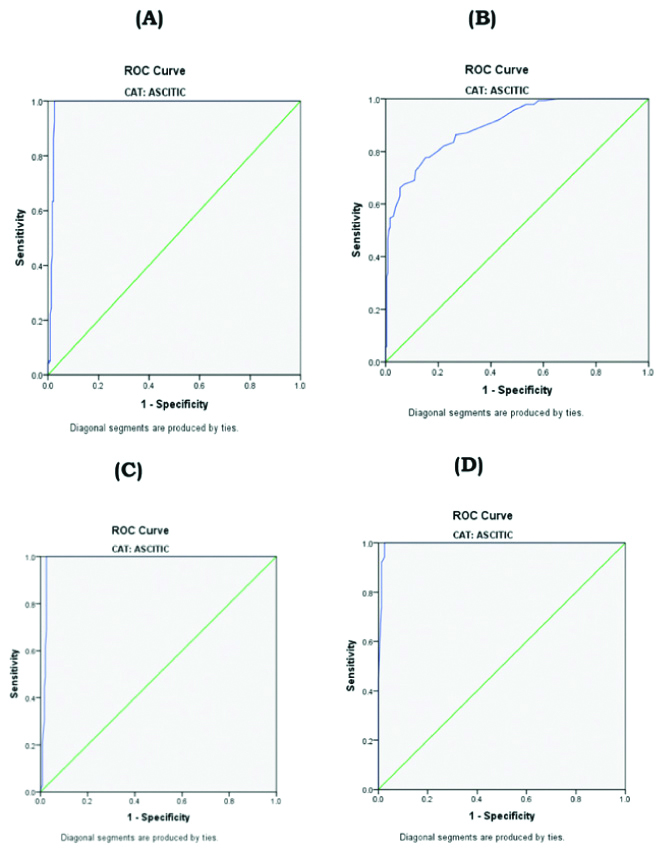
The study subjects were divided into Liver cirrhosis (n=230), 178 males and 52 females, age 20-75 years, (mean age 46.59±10.82); Ovarian cancer (n=145) with age 19-84 years (mean age 49.85±11.75) and Controls (n=150), 91 males and 59 females, age 24-65 years, (mean age 43.31±8.51). The results (Mean±SD) in the serum samples of two groups were analysed separately and compared with healthy controls, whereas in the ascitic fluid results were compared among the groups. In the present study, there was a significantly increased level of ascitic fluid TP, ALB, CHOL and TG were observed in ovarian cancer patients when compared to that of liver cirrhosis (p<0.001). The mean serum concentrations of the respective parameters were grossly decreased in liver cirrhosis patients when compared to that of ovarian cancer and healthy controls (p<0.001). The results are depicted in [Table/Fig-1,2] and cutoff values for ascitic fluid CHOL, TG, TP, ALB, SACG, and SATG were also derived in by using ROC curves and are depicted in [Table/Fig-3]. Receiver Operating Characteristics (ROC curve) showing sensitivity and specificity of biochemical parameters in the ascitic fluid were depicted in [Table/Fig-4,5]. The cutoff values obtained by ROC curves for ascitic fluid cholesterol was (67 mg %), Triglycerides (43.5 mg/dL), total protein (3.2 gm%), albumin (1.65 gm%), SAAG (1.11 gm%), SACG (65.5 mg%) and SATG (34.5 mg/dL). Ascitic fluid total proteins, albumin and SAAG had the highest diagnostic efficiency (99%) of all parameters, with a sensitivity of (100%) and specificity of 99%. Among all the parameters, SACG had the lowest sensitivity (64%) and diagnostic efficiency (61%). Ascitic fluid CHOL and SATG had the diagnostic efficiency 95%; 87% and sensitivity of 93%; 84%. The area under the curve of CHOL and SATG strongly indicated the diagnostic efficiency of 0.984 and 0.945, respectively.
Serum and Ascitic fluid concentrations of Total Proteins (TP), Albumin (ALB), Cholesterol (CHOL) and Triglycerides (TG) in ovarian cancer (n=145); liver cirrhosis (n=230); and control (n=150). p-value <0.05 is considered as statistical significant; C indicates (control serum); OC (ovarian cancer); LC (liver cirrhosis).
| Parameters | Serum (Mean±SD) | p-value | Ascitic fluid (Mean±SD) | p-value |
|---|
| TP (gm/dL) | Ovarian cancer | 6.67±0.60 | C & OC vs. LC <0.001 | 5.51±0.75 | <0.001 |
| Liver cirrhosis | 5.11±0.68 | 1.15±0.51 |
| Control | 6.88±0.39 | -- | -- |
| ALB (gm/dL) | Ovarian cancer | 3.76±0.51 | C & OC vs. LC <0.001 | 3.18±0.52 | <0.001 |
| Liver cirrhosis | 2.30±0.45 | 0.35±0.19 |
| Control | 4.01±0.38 | -- | -- |
| CHOL (mg/dL) | Ovarian cancer | 153.0±25.91 | C & OC vs. LC <0.001; OC vs. LC <0.001 | 87.32±17.73 | <0.001 |
| Liver cirrhosis | 89.53±20.11 | 15.50±5.81 |
| Control | 157.58±23.80 | -- | -- |
| TG (mg/dL) | Ovarian cancer | 124.84±34.40 | C & OC vs. LC <0.001; OC vs. LC <0.001 | 63.73±22.78 | <0.001 |
| Liver cirrhosis | 57.70±12.69 | 36.05±8.17 |
| Control | 135.96±36.12 | -- | -- |
Serum ascitic albumin gradient (SAAG), Serum ascitic cholesterol gradient (SACG) and Serum ascitic triglycerides gradient (SATG) in ovarian cancer and liver cirrhosis patients.
| Parameters | N | Mean±SD | p-value |
|---|
| SAAG | Ovarian cancer | 145 | 0.56±0.23 | <0.001 |
| Liver cirrhosis | 230 | 1.94±0.45 |
| SACG | Ovarian cancer | 145 | 65.60±23.74 | <0.05 |
| Liver cirrhosis | 230 | 73.98±19.08 |
| SATG | Ovarian cancer | 145 | 61.07±23.26 | <0.001 |
| Liver cirrhosis | 230 | 21.75±8.74 |
p-value <0.05 is considered as statistical significant;
Ascitic Fluid Diagnostic value of individual parameters in differentiating ovarian cancer from liver cirrhosis ascitic patients.
| Parameter | Cutoff % | Area under curve | Sensitivity (%) | Specificity (%) | Diagnostic efficiency (%) |
|---|
| CHOL (mg%) | 67 | 0.984 | 93 | 97 | 95 |
| TG (mg%) | 43.5 | 0.898 | 81 | 80 | 80.5 |
| TP (gm%) | 3.2 | 0.982 | 100 | 98 | 99 |
| ALB (gm%) | 1.65 | 0.994 | 100 | 98 | 99 |
| SAAG (gm%) | 1.11 | 0.996 | 100 | 99 | 99.5 |
| SACG (mg%) | 65.5 | 0.618 | 64 | 58 | 61 |
| SATG (mg%) | 34.5 | 0.945 | 84 | 91 | 87 |
TP: Total proteins; ALB: Albumin; CHOL: Cholesterol; TG Triglycerides; SAAG: Serum ascitic albumin gradient; SACG: Serum ascitic cholesterol gradient; SATG: Serum ascitic triglycerides gradient
Receiver operating characteristics (ROC curve) showing sensitivity and specificity of biochemical analytes in ascitic fluid, (A) SAAG vs SACG; (B) SAAG vs. SATG.
Receiver operating characteristics (ROC curve) showing sensitivity and specificity of biochemical parameters in ascitic fluid: (A) Cholesterol; B) Triglycerides; (C) Total Proteins; (D) Albumin.
Discussion
In the present study, there was a significantly increased level of ascitic fluid cholesterol and obtained at a cutoff value of 67 mg/dL with a sensitivity 93% and specificity of 97% in ovarian cancer patients, whereas in the study of Castaldo G et al., and Banerjee M et al., had cited a sensitivity of 40-60% for the cytological presence of malignant cells in the ascitic fluid [9,18]. In the previous studies different authors had established variation in their cutoff values for ascitic fluid cholesterol in various malignant conditions, Gerbes AL et al., (45 mg/dL) with sensitivity 90% and specificity 82% [19]. Vyakaranam S et al., (2011) (62 mg%), Gupta R et al., (>55 mg%), a diagnostic accuracy of 94%, Sharath Chandra LK et al., (>67 mg%) had a diagnostic accuracy of 96%, Rana SV et al., (>70 mg%) had diagnostic accuracy of 94% [7,20-22]. The variations could be due to the variable population, serum cholesterol levels and the extent of peritoneal implants.
In the study by Gerbes AL et al., showed, cholesterol is a sensitive parameter for the differential diagnosis of malignant ascites [19]. In this study, cutoff value of ascitic fluid cholesterol was 67 mg% with the highest sensitivity and specificity than the previous studies. Hence, it strongly supports the ascitic fluid cholesterol can be utilised as an additional biochemical marker for the screening of ovarian cancer.
The pathogenesis of increased levels of cholesterol in the malignant ascitic fluid was not completely explained. However, previous authors explained increased levels of cholesterol might be due to increased vascular permeability [23]; endothelial permeability [24]; releasing from neoplastic cells [25]; disintegration of many cells of primary tumours which irritates peritoneal serosa leading to increased permeability of the carcinomatous membrane [26]; increased cholesterol synthesis [27], the increased levels of cholesterol in the abdominal fluid agreeing upon hypothesis of blockage lymphatic drainage [28].
In the present study, there were significantly increased levels of ascitic fluid TG in ovarian cancer patients when compared to liver cirrhosis patients and at a cutoff value of 43.5 mg% with a sensitivity of 81% and specificity of 80%. The increased levels of triglycerides in ovarian cancer ascitic fluid patients may be derived from plasma, but chylomicrons of intestinal origin may contribute to the total amount of triglycerides in ascitic fluid.
In this study, significantly increased levels of ascitic fluid total proteins and albumin in ovarian cancer patients with a cutoff value of 3.2 gm% and 1.65 gm% with a sensitivity of 100% and specificity of 98%, respectively. The present study is in corroboration with the previous studies [9,23,26]. In the present study SAAG had a cutoff value of 1.11 gm% with a sensitivity of 100% and specificity of 99%. In prospective studies, SAAG has shown to be the best single test for classifying ascites than the older criterion (transudate versus exudate) [7,8,10,29].
In this study, SACG had a cutoff value of 65.5 mg% with a sensitivity of 64% and specificity of 58%, which had the lowest sensitivity and diagnostic efficiency whereas Vyakaranam S had a cutoff value of 53% with 90% sensitivity and 95% specificity [7]. Unlike ascitic fluid cholesterol, SACG could not differentiate ovarian cancer from liver cirrhosis patients.
Limitation(s)
The advanced molecular techniques and methodologies were the limitation of this study. Moreover, to study and explore the underlying mechanism, molecular markers like VEGF, VPF, telomerase activity, fibronectin would have been more helpful in the differential diagnosis of ascitic patients.
Conclusion(s)
This study showed significantly increased levels of ascitic fluid cholesterol and SATG in ovarian cancer patients when compared to that of liver cirrhosis (p<0.001). SATG with a cutoff value of 34.5 mg/dL has a sensitivity of 84%, specificity of 91% and area under curve 0.945. Therefore, the present study suggested, ascitic fluid cholesterol and SATG is a more sensitive parameter that would be used as an additional biomarker along with SAAG for screening and differential diagnosis of ovarian cancer, in combination with clinical, pathological and imaging data.
p-value <0.05 is considered as statistical significant;
TP: Total proteins; ALB: Albumin; CHOL: Cholesterol; TG Triglycerides; SAAG: Serum ascitic albumin gradient; SACG: Serum ascitic cholesterol gradient; SATG: Serum ascitic triglycerides gradient