Introduction
Pregnancy is a period that is characterised by remarkable physiological changes which are needed to support the growing fetus. Micronutrients play a crucial role in the maintenance of pregnancy. Among the micronutrients, magnesium has got ample amount of clinical relevance to pregnancy. Magnesium deficiency has been associated with reproductive risk during pregnancy such as anaemia, pre-eclampsia, eclampsia, fetal growth retardation, preterm labour, low intrauterine growth rate and leg cramps.
Aim
To find out whether there was significant difference in the magnesium levels among pregnant women with and without pregnancy related complications and also to find out whether the magnesium levels differed significantly among the three trimesters.
Materials and Methods
This was a cross-sectional study done among 240 pregnant patients from different trimesters attending the Obstetrics and Gynaecology Outpatient Department. Out of them 164 women had complications like pre-eclampsia, Gestational Diabetes Mellitus, leg cramps and history of abortions. Demographic details were obtained from all the women. BMI was calculated for each subject using the formula BMI=weight(kg)/height(m2). Haemoglobin was assessed in all samples using cyanmethaemoglobin method. Magnesium levels were analysed in the all samples using automated analyser in the Biochemistry laboratory. Student’s t-test was used to compare the levels of magnesium among the groups with and without complications. ANOVA test was used to compare the three trimesters.
Results
The pregnant women were divided into three groups based on the different trimesters in which the samples were taken. Magnesium levels among the women in the first trimester were (2.96±0.83) second trimester (2.99±1.48) and third trimester (3.05±1.48) respectively. Women with previous abortions were found to have less magnesium levels (2.71) compared to women without a history of abortion (3.11) and value was found to be statistically significant (p<0.007) and magnesium levels among vegetarians (2.45) were lower than non vegetarians (3.08) and it was found to be statistical significant (p<0.013). There was no significant difference in the magnesium levels among pregnant women with complications and those without complications.
Conclusion
The study shows the importance of maintaining the magnesium levels among pregnant women. There was no difference in the magnesium levels among the three trimesters. Study also points out the influence of parity, diet and occupation of pregnant women on the levels of magnesium.
Gestational diabetes mellitus, Micronutrients, Pre-eclampsia, Trimesters
Introduction
Maternal nutrition plays a pivotal role for a good pregnancy outcome. Pregnancy is a condition which is associated with increased requirement of all types of nutrients. Maternal nutrient stores supply all the macronutrients and micronutrients required for the proper development of the growing fetus [1].
Pregnancy is associated with a stress on nutritional reserve with depletion of essential nutrients like iron, folic acid, vitamins and trace minerals. Improved maternal nutrition is associated with decreased pregnancy complications like pre-eclampsia, gestational diabetes mellitus, intrauterine growth retardation which in turn affects the long term morbidity and mortality of the mother and fetus [2].
There is a complex interrelationship between the various trace elements and the development of various maternal complications. Appreciating the relation between maternal serum levels of these elements and birth outcomes could offer a base for developing nutritional interventions that will progress birth outcomes and long term superiority of life and reduce mortality, morbidity and healthcare costs [3].
India is a developing country where the prevalence of maternal complications is more but studies related to this is less. A study done by Kotecha PV showed that 70% of the population in India had 50% lack of micronutrients [4]. The importance of trace elements in pregnancy is still underestimated though many studies prove that trace element deficiency is associated with development of adverse pregnancy outcomes. Magnesium deficiency has been associated with the development of pre-eclampsia and intrauterine growth retardation, preterm labour [4,5].
Magnesium is an essential trace mineral required for the proper functioning of our body. Magnesium helps in the action of many enzymes to regulate the different functions in our health like temperature regulation, synthesis of nucleic acid, proteins and maintain the membrane of nerves and muscles [6,7].
The Recommended Daily Allowance for magnesium among pregnant women in the age group 14-18 years is 400 mg/day and those in the age group of 19-30 years are 350 mg/day and among the age group 30-40 years it is 400 mg/day [8]. Green leafy vegetables, whole milk, paneer, cashew nuts are few rich sources of magnesium [9].
Magnesium deficiency during pregnancy can produce maternal, fetal, and paediatric consequences that can last for a lifetime. Studies done in animals have shown to have marked effects on parturition and postuterine involution. It is found to affect fetal growth and development, cause haematological effects and turbulence in temperature regulation and teratogenic effects. Premature labour can occur due to increased uterine hyperexcitability leading to preterm birth [10].
Most of the laboratories have standard reference intervals for all the parameters for healthy men and women but separate reference interval in pregnancy is not available. The biochemical changes occurring in pregnancy influence the values of almost all the laboratory parameters. Magnesium being an important micronutrient in pregnancy, this study was conducted to find out whether there was significant difference in the magnesium levels among pregnant women with and without pregnancy related complications and also to find out whether the magnesium levels differed significantly among the three trimesters.
Objectives:
Determine the difference in magnesium levels among working pregnant women and housewives.
Determine the levels of magnesium among pregnant women who are vegetarian (vegans) and non vegetarian (vegans).
Determine the correlation between BMI and magnesium among the pregnant women
Materials and Methods
This cross-sectional study was conducted from December 2017 to March 2018. Permission from Institutional Ethical Committee was got before starting the study (Proposal No: 350/IHEC/10-17). Blood samples were collected from 240 pregnant women who attended the Obstetrics and Gynaecology Department of Chettinad Hospital and Research Institute. Among them three patients had pre-eclampsia, 14 had gestational diabetes mellitus, 29 had hypothyroidism, 56 women complained of leg cramps, 62 had previous history of abortions. All subjects were explained about the purpose of the study and if willing to participate, informed consent was obtained from all the participants in the study. The power of the study was 80% with 90% confidence interval. Demographic details were collected from all the subjects.
Pre-eclampsia diagnosed as BP levels more than 140/90 mm of Hg with proteinuria, Gestational Diabetes Mellitus (diagnosed by Oral Glucose Tolerance Test (OGTT) by Diabetes in Pregnancy Study Group of India guidelines) [11] thyroid disorders (abnormal thyroid hormone levels), anaemia (Hb levels less than 11g.dl) and frequent episodes of leg cramps. Personal history was taken. Height and weight were measured for each candidate and Body Mass Index (BMI) was calculated using the formula BMI=weight (kg)/height (m2). Details regarding Socio-economic status, dietary pattern, previous obstetric history were obtained.
All pregnant women of the age group 18-35 years attending the Obstetric and Gynaecology OPD were included in the study. Subjects with endocrine disorders, kidney disease, peptic ulcer disease, chronic hypertension, history of intrauterine growth restriction, acquired immune deficiency syndrome, sickle cell anaemia, retroviral infections were excluded from the study.
Peripheral venous blood (5 mL) was collected each in red top vaccutainer (BD vacationer) for estimating magnesium levels and 5 ml in violet colored vaccutainer for estimating the amount of haemoglobin. After extraction, the sample in red topped vaccutainer was centrifuged at 2000 rpm for five minutes to separate the serum and was stored at -80 degree celcius for further analysis. Violet topped vaccutainer was sent to pathology laboratory for estimation of haemoglobin.
Magnesium can be analysed in blood using methods like Atomic absorption spectrophotometry, Ion selective electrodes and by using semi autoanalyser, but in all these methods, there will be interferences from other minerals and is less sensitive. In this study we have analysed magnesium levels in serum using auto-analyser [12]. Magnesium was analysed using the Methylthymol blue method and was run in Seimens Dimension RXL automated machine in the Biochemistry laboratory. Increased haemoglobin levels, bilirubin levels, and lipemic samples can falsely increase the magnesium levels in serum. Haemoglobin was analysed using cyan methhaemoglobin method in the Pathology laboratory in Beckman coulter automated machine.
Statistical Analysis
Statistical analysis has been carried out using SPSS (Statistical Package for Social Sciences. (IBM version 21.0) software. The p-value less than 0.05 was considered statistically significant. Continuous variables were presented in the form of mean±SD and categorical variables were presented in the form of percentages. Pearsons correlation analysis was done to find out the correlation between magnesium, age, and BMI. Student’s t-test was used to compare the levels of magnesium among the groups with and without complications. ANOVA test was used to compare the three trimesters. ANOVA test was done to compare the mean of the continuous variables among the women in the first, second and third trimesters of pregnancy.
Results
Among the 240 women enrolled for the study, more than 50% of the women had an educational qualification above higher secondary grade. About 85.83% of the women were housewives and 14.17% were working women. A 53.75% were primi, 33.3% were in second gravida, 10.42% in the third gravida and 2.5% in the fourth gravida. A 25.83% of women had previous history of abortion and 74.17% of women did not had any history of abortion [Table/Fig-1]. The 240 patients were divided into three groups based upon the trimesters in which they presented. The Mean values for magnesium among the first trimester patients were 2.96±0.83, for second trimester 2.99±1.48, and third trimester 3.05±1.48. ANOVA analysis showed that magnesium level showed no significant difference in between three groups [Table/Fig-2].
Demographic data of the subjects.
| Variables | No. | % |
|---|
| Education | Up to high school | 52 | 21.67 |
| Sr. Sec. | 42 | 17.50 |
| Degree/Diploma | 85 | 35.42 |
| PG | 39 | 16.25 |
| Professional | 22 | 9.17 |
| Occupation | Housewife | 206 | 85.83 |
| Working | 34 | 14.17 |
| Pregnancy | Primi | 129 | 53.75 |
| Second gravida | 80 | 33.33 |
| Third gravida | 25 | 10.42 |
| Fourth gravida | 6 | 2.50 |
| H/O Abortion | Yes | 62 | 25.83 |
| No | 178 | 74.17 |
| Trimester | First | 85 | 35.42 |
| Second | 79 | 32.92 |
| Third | 76 | 31.67 |
A one-way analysis of continuous variables among three trimesters (p<0.05-statistically significant).
| Variables | Trimester | One-way ANOVA |
|---|
| First | Second | Third |
|---|
| Mean | SD | Mean | SD | Mean | SD | F-value | p-value |
|---|
| Age | 25.56 | 3.32 | 25.68 | 3.72 | 29.24 | 27.80 | 1.342 | 0.263 |
| Height (cm) | 156.78 | 6.46 | 157.53 | 5.11 | 157.26 | 6.98 | 0.312 | 0.732 |
| Weight (Kg) | 60.91 | 9.11 | 63.14 | 9.74 | 63.42 | 11.28 | 1.545 | 0.215 |
| BMI (Kg/m2) | 24.80 | 3.58 | 25.49 | 4.05 | 25.73 | 4.76 | 1.096 | 0.336 |
| Systolic BP (mm of Hg) | 110.71 | 9.05 | 111.44 | 7.90 | 111.22 | 9.22 | 0.154 | 0.857 |
| Diastolic BP (mm of Hg) | 71.55 | 8.37 | 70.78 | 6.90 | 71.55 | 7.98 | 0.257 | 0.773 |
| Hb level (g/dL) | 11.09 | 0.79 | 11.42 | 1.14 | 11.15 | 1.32 | 0.863 | 0.425 |
| Magnesium Level (mg/dL) | 2.96 | 0.83 | 2.99 | 1.48 | 3.05 | 1.48 | 0.110 | 0.896 |
BMI: Body mass index; Hb: Haemoglobin
Magnesium levels in working women were found to be higher (3.12 mg/dL) than housewives (2.98 mg/dL) but it was not statistically significant. BMI was found to be 26±3.4 among working women and 28.4±4.22 among housewife. Haemoglobin levels were 10.82±1.11 among working women and 11.31±1.13 among house wives [Table/Fig-3].
Independent sample T-test comparing BMI, haemoglobin and magnesium levels in working women and housewives.
| Variables | Occupation | Independent samples t-test |
|---|
| Working | Housewife |
|---|
| Mean | SD | Mean | SD | t-value | p-value |
|---|
| BMI (Kg/m2) | 26 | 3.4 | 28.4 | 4.22 | 0.81 | 0.21 |
| Hb Level (g/dL) | 10.82 | 1.11 | 11.31 | 1.13 | -1.673 | 0.097 |
| Magnesium level (mg/dL) | 3.12 | 0.94 | 2.98 | 1.33 | 0.599 | 0.550 |
Women with previous abortions were found to have less magnesium levels (2.71 mg/dL) compared to women without a history of abortion (3.11 mg/dL) and value was found to be statistically significant (p<0.007) [Table/Fig-4].
Independent sample T-test comparing the magnesium, BMI and haemoglobin levels among women with and without history of abortion.
| Variables | Abortion | Independent samples t-test |
|---|
| Yes | No |
|---|
| Mean | SD | Mean | SD | t-value | p-value |
|---|
| BMI (kg/m2) | 25.85 | 4.56 | 25.12 | 3.98 | 1.191 | 0.235 |
| Hb Level (g/dL) | 11.24 | 1.35 | 11.23 | 1.07 | 0.009 | 0.992 |
| Magnesium level (mg/dL) | 2.71↓ | 0.78 | 3.11← | 1.40 | -2.743 | 0.007* |
BMI: Body mass index; Hb: Haemoglobin; *p<0.05-statistically significant
Magnesium levels among vegans (2.45) were lower than non vegans (3.08) and it was found to be statistical significant (p=0.013). BMI was found to be 24.57±3.37 among vegans and 25.43±4.23 among non vegetarians. Haemoglobin levels were 10.89±1.40 among vegans and 11.26±1.11 among non vegetarians [Table/Fig-5].
Independent sample t-test comparing the magnesium, BMI and haemoglobin level among vegan and non vegans.
| Variables | Diet | Independent samples t-test |
|---|
| Vegetarian | Non vegetarian |
|---|
| Mean | SD | Mean | SD | t-value | p-value |
|---|
| BMI (kg/m2) | 24.57 | 3.37 | 25.43 | 4.23 | -1.061 | 0.290 |
| Hb level (g/dL) | 10.89 | 1.40 | 11.26 | 1.11 | -0.934 | 0.352 |
| Magnesium level (mg/dL) | 2.45↓ | 0.76 | 3.08← | 1.32 | -2.517 | 0.013* |
BMI: Body mass index; Hb-Haemoglobin; *p<0.05-statistically significant
In this study, it was found that as the parity of the women increased magnesium level decreased, highest value was found among the primigravida (3.02) but there was no significant difference between the groups [Table/Fig-6].
One-way analysis showing the magnesium, BMI and haemoglobin levels among different parity.
| Variables | Pregnancy | One-way |
|---|
| 1 | 2 | 3 | 4 | ANOVA |
|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F-value | p-value |
|---|
| BMI (kg/m2) | 24.96 | 4.08 | 25.68 | 3.95 | 25.35 | 3.40 | 28.28 | 8.55 | 1.575 | 0.196 |
| Hb level (g/dL) | 11.25 | 1.11 | 11.27 | 1.07 | 11.11 | 1.53 | 10.94 | 1.09 | 0.174 | 0.914 |
| Magnesium level (mg/dL) | 3.02 | 1.06 | 3.01 | 1.40 | 2.92 | 1.87 | 2.58 | 1.30 | 0.254 | 0.858 |
BMI: Body mass index; Hb: Haemoglobin
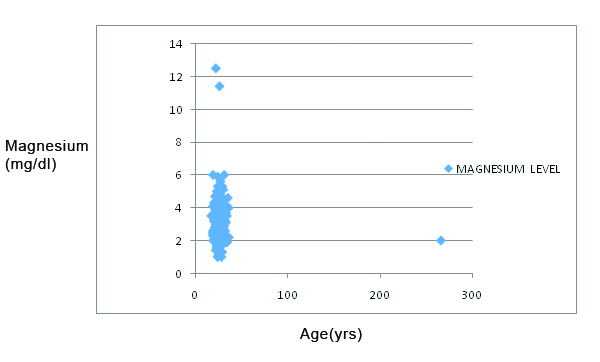
Pearson correlation analysis done between magnesium and age showed a negative correlation coefficient of -0.064, which was not statistically significant [Table/Fig-7].
Pearson’s correlation between age and magnesium levels among pregnant women.
X axis: Age in years; Y axis: Magnesium levels (mg/dL)
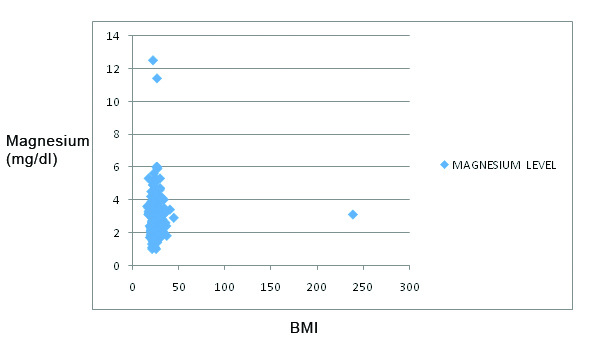
Pearsons correlation analysis between magnesium and BMI showed a negative correlation coefficient of -0.0016 which was not statistically significant [Table/Fig-8].
Pearson’s correlation between BMI and magnesium levels among pregnant women.
X axis: BMI; Y axis: Magnesium levels (mg/dL)
There was no significant difference in the magnesium levels among pregnant women with complications and those without complications [Table/Fig-9].
Independent sample t-test comparing the magnesium level between normal pregnant women and with complication.
| Variables | Magnesium level in | Independent samples t-test |
|---|
| Pregnant women with complication | Pregnant women without complication |
|---|
| Mean | SD | Count | Mean | SD | Count | t-value | p-value |
|---|
| Diabetes mellitus | 2.27 | 0.23 | 3 | 3.01 | 1.29 | 237 | -0.995 | 0.321 |
| GDM | 3.04 | 1.34 | 14 | 3.00 | 1.28 | 226 | 0.112 | 0.911 |
| Thyroid | 2.83 | 0.90 | 29 | 3.02 | 1.33 | 211 | -0.749 | 0.455 |
| Pre-eclampsia | 2.47 | 0.64 | 3 | 3.01 | 1.29 | 237 | -0.722 | 0.471 |
| Anaemia | 2.86 | 1.11 | 10 | 3.00 | 1.29 | 230 | -0.348 | 0.728 |
Discussion
Magnesium has established role in obstetrics, it being an essential element to fetal well-being. Deficiency of magnesium may be possibly associated with pre-eclampsia, abortion, preterm birth, leg cramps [13]. The formation of new tissues (maternal and fetal) during pregnancy requires high magnesium intakes than normal women of comparable age [14].
In present study, there was no significant difference in the mean levels of magnesium among the first, second and third trimesters. In a study done by Shaikh K et al., out of 150 patients, 60 patients in second trimester and 40 patients in the third trimester had low magnesium values (below reference range of 1.8 mEq/L), remaining had serum magnesium level within normal range of 1.8-2.9 mg/dL [15].
Larsson A et al., found that magnesium reference range was 1.2-2.3 mg/dL. They divided the patients into six groups based on gestational age as 7-17 weeks (1.6-1.7 mg/dL), 17-24 weeks (1.5-1.6 mg/dL), 24-28 weeks (1.5-1.53 mg/dL), 28-31 weeks (1.5-1.55 mg/dL), 31-34 weeks (1.55 mg/dL), 34-38 weeks (1.2-1.5 mg/dL) (149), but the drawback of the study was that the study population was very less (52 patients) [16].
Pathak P et al., showed that among 259 pregnant women, 113 had lower serum magnesium levels (<1.8mg/dL) when compared with normal healthy control patients [17]. They estimated serum magnesium by the standard atomic absorption spectrophotometric method. Atomic absorption spectrophotometer is considered as the best method for the estimation of serum magnesium [18], so that may be the cause for the disparity in the values.
The present study shows a decreased level of magnesium among women who had a history of abortion (2.71) compared to women who did not had a past history of abortion (3.11) and it was found to be statistically significant (p<0.007). Magnesium deficiency during pregnancy may interfere with fetal growth and development and has proven to cause a range of morbidity from haematological to teratogenic damages [19]. Magnesium promotes myometrial activity by modulating calcium uptake, binding and distribution in smooth muscle cells, hence magnesium deficiency during pregnancy can lead to increased uterine excitability [20].
Alnakash AH et al., conducted a study on 82 pregnant patients and their serum magnesium level was significantly low (p-value <0.012) in the women who had a history of abortion (1.597) than the control group (1.682) [21].
In present study, pregnant women who are non vegetarians had a more mean magnesium level (3.08) than the vegans (2.45). Magnesium levels are less in the food consumed by vegetarians and those belonging to the low socioeconomic groups than non vegetarians and those belonging to higher socio economic groups. Diet rich in protein increases magnesium absorption. Socioeconomic status affected serum magnesium levels significantly in term as well as preterm group, the values being lowest for women belonging to low socioeconomic status. These observations are similar to those reported by Franz KB and Potnis AV et al., [22,23].
Sharma A et al., showed that non vegetarians had a high serum magnesium levels (3.0) when compared to vegan pregnant women (2.29) [24] which is on par with our results.
Limitation(s)
The sample size of the study is small. Another limitation is that only one trace element was analysed in this study.
Conclusion(s)
The present study shows that there is no significant difference in the magnesium levels among women in the three trimesters. It also points out that dietary patterns, socioeconomic status and parity of women play an important role in maintaining the levels of magnesium among pregnant women. This study points out the importance of maintaining proper levels of magnesium among pregnant women.
BMI: Body mass index; Hb: Haemoglobin
BMI: Body mass index; Hb: Haemoglobin; *p<0.05-statistically significant
BMI: Body mass index; Hb-Haemoglobin; *p<0.05-statistically significant
BMI: Body mass index; Hb: Haemoglobin
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 21, 2020
Manual Googling: Jul 06, 2020
iThenticate Software: Aug 17, 2020 (11%)
[1]. Ugwuja EI, Akubugwo EI, Ibiam U, Obodoa O, Ugwu N, Plasma copper and zinc among pregnant women in Abakaliki, Southeastern NigeriaInternet J Nutr Wellness 2010 10:110.5580/b7d [Google Scholar] [CrossRef]
[2]. Roberts CT, IFPA Award in Placentology Lecture: Complicated interactions between genes and the environment in placentation, pregnancy outcome and long term healthPlacenta 2010 31:S47-53.10.1016/j.placenta.2010.01.00120096927 [Google Scholar] [CrossRef] [PubMed]
[3]. Abu-Saad K, Fraser D, Maternal nutrition and birth outcomesEpidemiologic Reviews 2010 32(1):05-25.10.1093/epirev/mxq00120237078 [Google Scholar] [CrossRef] [PubMed]
[4]. Kotecha PV, Micronutrient malnutrition in India: Let us say “no” to it nowIndian journal of community medicine: Official publication of Indian Association of Preventive & Social Medicine 2008 33(1):910.4103/0970-0218.3923519966988 [Google Scholar] [CrossRef] [PubMed]
[5]. Fall CH, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJ, Micronutrients and fetal growthThe Journal of Nutrition 2003 133(5):1747S-56S.10.1093/jn/133.5.1747S12730494 [Google Scholar] [CrossRef] [PubMed]
[6]. Zarean E, Tarjan A, Effect of magnesium supplement on pregnancy outcomes: A randomized control trialAdvanced Biomedical Research 2017 6:10910.4103/2277-9175.21387928904937 [Google Scholar] [CrossRef] [PubMed]
[7]. McNamara HC, Crowther CA, Brown J, Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labourCochrane Database of Systematic Reviews 2015 (12)10.1002/14651858.CD011200.pub226662716 [Google Scholar] [CrossRef] [PubMed]
[8]. National Institutes of Health. Magnesium Fact Sheet for Health Professionals. Version current. Available on http://accurateclinic.com/wp-content/uploads/2016/07/Magnesium-%E2%80%94-Health-Professional-Fact-Sheet.pdf. Last assessed 6th July [Google Scholar]
[9]. Institute of Medicine (US). Subcommittee on Nutritional Status, Weight Gain during Pregnancy, Institute of Medicine (US). Subcommittee on Dietary Intake, Nutrient Supplements during Pregnancy. Nutrition during pregnancy: Part I, weight gain: Part II, nutrient supplements. Natl Academy Pr; 1990 Jun 15 [Google Scholar]
[10]. Durlach J, New data on the importance of gestational Mg deficiencyJ Am Coll Nutr 2004 23(6):694S-700S.10.1080/07315724.2004.1071941115637217 [Google Scholar] [CrossRef] [PubMed]
[11]. Ising H, Bertschat F, Günther T, Jeremias E, Jeremias A, Measurement of free magnesium in blood, serum and plasma with an ion-sensitive electrodeEur J Clin Chem Clin Biochem 1995 33(6):365-371.10.1515/cclm.1995.33.6.3657578617 [Google Scholar] [CrossRef] [PubMed]
[12]. Mishra S, Bhadoria AS, Kishore S, Kumar R, Gestational diabetes mellitus 2018 guidelines: An updateJ Family Med Prim Care 2018 7(6):116910.4103/jfmpc.jfmpc_178_1830613492 [Google Scholar] [CrossRef] [PubMed]
[13]. Carroli G, Rooney C, Villar J, How effective is antenatal care in preventing maternal mortality and serious morbidity? An overview of the evidencePaediatric and perinatal Epidemiology 2001 15:01-42.10.1046/j.1365-3016.2001.0150s1001.x11243499 [Google Scholar] [CrossRef] [PubMed]
[14]. Seelig MS, The role of magnesium in normal and abnormal pregnancyIn Magnesium Deficiency in the Pathogenesis of Disease 1980 Boston, MASpringer:29-50.10.1007/978-1-4684-9108-1_2 [Google Scholar] [CrossRef]
[15]. Shaikh K, Das CM, Baloch GH, Abbas T, Fazlani K, Jaffery MH, Magnesium associated complications in pregnant womenWorld App Sci J 2012 17(9):1074-78. [Google Scholar]
[16]. Larsson A, Palm M, Hansson LO, Axelsson O, Reference values for clinical chemistry tests during normal pregnancyBJOG: An International Journal of Obstetrics & Gynaecology 2008 115(7):874-81.10.1111/j.1471-0528.2008.01709.x18485166 [Google Scholar] [CrossRef] [PubMed]
[17]. Pathak P, Kapoor SK, Kapil U, Dwivedi SN, Serum magnesium level among pregnant women in a rural community of Haryana State, IndiaEur J Clin Nutr 2003 57(11):150410.1038/sj.ejcn.160183214576766 [Google Scholar] [CrossRef] [PubMed]
[18]. Denis W, The determination of magnesium in blood, plasma, and serumJ Bio Chem 1922 52(2):411-15.10.1016/S0021-9258(18)85835-5 [Google Scholar] [CrossRef]
[19]. Makrides M, Crowther CA, Magnesium supplementation in pregnancyCochrane Database of Systematic Reviews 2001 4:CD00093710.1002/14651858.CD000937 [Google Scholar] [CrossRef]
[20]. Durlach J, Pagès N, Bac P, Bara M, Guiet-Bara A, Beta-2 mimetics and magnesium: are true or false friends?Magnes Res 2003 16:218-33. [Google Scholar]
[21]. Alnakash AH, Hamood MA, NAbdullah T, Ghafoor YA, A study of serum magnesium and calcium levels in missed abortionSynthesis 2013 7:8 [Google Scholar]
[22]. Franz KB, Magnesium intake during pregnancyMagnesium 1987 6(1):18-27. [Google Scholar]
[23]. Potnis AV, Patel PV, Purandare SN, Magnesium, the ignored, element during pregnancyJ Obstet Gynec India 1977 27:343-45. [Google Scholar]
[24]. Sharma A, Kharb S, Gulati N, Serum magnesium levels in preterm labour in relation to socioeconomic statusJ Clin Biochem 1998 13(2):123-25.10.1007/BF0286787423105193 [Google Scholar] [CrossRef] [PubMed]