Ovarian epithelial cancer is an aggressive malignancy which has a highly invasive and metastatic feature with the reported 5-year survival only 37% [1].
OSC is a common ovarian epithelial malignancy, which now graded as low-grade and high-grade [2]. The evolution of aggressive carcinoma, occurs in a slowly step-wise manner. Low-grade neoplasm are slowly growing and have better prognosis than high-grade neoplasm [3].
The insulin-like growth factor mRNA-binding protein 3 (IMP3) is one of mRNA-binding protein family which regulates mRNA transport, translation and turnover [4]. Previous studies have concluded that IMP3 is an onco-fetal protein that facilitates the invasive abilities of tumour cells [5]. IMP3 was found to be overexpressed in a variety of human cancers, including ovarian, lung and gastrointestinal carcinomas [6,7]. Being associated with advanced tumour stage and adverse prognostic outcome in these cancers and which makes this protein a reliable biomarker to distinguish some cancers from their benign mimetic lesions [8].
Epithelial to Mesenchymal Transition (EMT) is the process by which epithelial cancer cells lose cell-cell adhesion, apical-basal polarity and acquire spindle cell morphology and gain mesenchymal markers positivity, which help them to metastasise [9]. Hypoxia [10], inflammation [11] and oxidation stress [12], are potent EMT inducers. These factors trigger signals that converge on EMT-inducing transcriptional factors such as Snail, Slug, Twist, with diminished expression of epithelial-related genes such as E-cadherin and, increased expression of mesenchymal-related genes such as vimentin [13]. The objectives of this study were to analyse IMP3 expression in serous ovarian tumours of Egyptian women, and to correlate the expression in malignant tumours with EMT related markers (E-cadherin and vimentin) and clinicopathological factors to assess the clinical application of IMP3 in the diagnosis, prognosis and possible application in targeted therapy of such tumours.
Materials and Methods
Type of Study and Specimen Collection
This was a cross-sectional research that was done at the Departments of Pathology, Obstetric and Surgery, Zagazig and Banha Universities Hospitals, Egypt. The research included fifty-nine cases of ovarian serous tumours (43 cases OSC, 6 cases border line tumour and 10 cases benign cystadenoma) that were collected from the archives of Pathology Departments from January 2014 to December 2018. All procedures performed in the current study were ethically approved by Institutional Review Board (ZU -#IRB approval number 4943).
Inclusion criteria: Female patients with primary ovarian tumour debulking operation, the specimens included were diagnosed by two pathologist and met the requirement of the Ethical Committee.
Exclusion criteria: Cases that received chemotherapy before operation or cases with lost clinical data were excluded from the study.
In each case, tissue samples were fixed by formalin and paraffin-embedded blocks were performed. The clinicopathological data were collected from the patients’ medical files.
Histopathological Evaluation
Four slides of 4 μm thickness were cut from each Paraffin block. The sections were dewaxed, then one slide was stained with haematoxylin and eosin and examined to confirm the previous diagnosis, asses the tumour grade according to the World Health Organisation classification (WHO) criteria 2016 [14]. Stage was defined according to the FIGO (The International Federation of Gynaecology and Obstetrics staging system) [15].
Immunohistochemical staining: For Immunohistochemical (IHC) assessment, 3 slides were mounted on positively charged slides. They were immune-stained for IMP 3 antibody, E-cadherin antibody and Vimentin antibody obtained from Thermo Fisher Scientific/Lab Vision Corporation, Rockford, and USA. IHC staining was performed, using universal detection kit (Thermo Scientific, USA) according to the manufacturer’s data in which 3,3’-Diaminobenzidine (DAB) was utilised as a chromogen. A negative control was used for each marker, by omitting the primary antibody. Antibodies details shown in [Table/Fig-1].
The markers of the study.
| Antibody | Type | State | Positive control | Incubation | Antigen retrieval |
|---|
| IMP3 | Rabbit polyclonal | Concentrated (1:100) | Tonsil | 1 hour | Citrate buffer |
| E-cadherin | Mouse monoclonal | Concentrated (1:100) | Skin | 1 hour | EDTA |
| Vimentin | Mouse monoclonal | Concentrated (1:50) | Human bladder tissue | 1 hour | EDTA |
EDTA: Ethylenediamine Tetra-acetic Acid; IMP3: Insulin-like growth factor-2 mRNA binding protein 3; E-cadherin: Epithelial cadherin
Immunohistochemical (IHC) Evaluation
IMP3 expression was detected as cytoplasmic dark brown staining of the cellular tumour. The proportion of positively stained cells was assessed by microscopic inspection of the whole stained tumour sections. The intensity of IMP3 staining was noted as weak, moderate, or strong. IMP3 expression was considered negative in absent staining or staining less than 5% of cellular tumour. The expression was considered positive in any definite cytoplasmic staining in more than 5% of cellular tumour, no matter the staining intensity [16].
Membranous E-cadherin and cytoplasmic vimentin expression was evaluated following the methods previously designated by [17] E-cadherin and vimentin was graded into groups from 0 to 3+ as follows: 0 (no staining); weak 1+ (1-25% staining); moderate +2 (26-50% staining); and strong +3 (>50% staining). For E-cadherin, the 2+ and 3+ groups were considered as positive. For vimentin, the 3+ groups were considered as positive [17].
Statistical Analysis
Analysis of the continuous variables was expressed as a means±standard deviation (SD). Analysis of categorical data was achieved using the Chi-square test (χ2) or Fisher’s-Exact test, Spearman correlation was done to detect the correlation between IMP3 and EMT related markers. The statistical studies were performed with SPSS software (version 19.0; SPSS, Chicago, IL). The p-values ≤0.05 were regarded as statistically significant.
Results
Clinicopathological Results
The mean age of the studied 59 ovarian serous tumours at initial surgery was 53.16±12.03 years (range 28-68 years). Among these 59 cases, 16.95% (10/59) of cases were benign cystadenomas, 10.17% (6/59) were borderline, and 72.88% (43/59) were OSC. Of the OSCs 53.49% (23/43) were low grade while 46.51% (20/43) were high grade. All carcinomas patients’ clinicopathological variables are presented in [Table/Fig-2].
Correlation of the clinicopathological parameters of the studied 43 OSC with IMP3 and EMT related markers expression.
| Clinico-pathological variables | n (%) 43 (100%) | IMP3 expression | E-cadherin expression | Vimentin expression |
|---|
| Negative n (%) | Positive n (%) | Negative n (%) | Positive n (%) | Negative n (%) | Positive n (%) |
|---|
| Age |
| ≤50 | 15 (34.88%) | 4 (26.7%) | 11 (73.3%) | 10 (66.7%) | 5 (33.3%) | 4 (26.7%) | 11 (73.3%) |
| >50 | 28 (65.11%) | 6 (21.4%) | 22 (78.6%) | 13 (46.4%) | 15 (53.6%) | 5 (17.9%) | 23 (82.1%) |
| *p-value | | 0.487 | 0.172 | 0.380 |
| Size of the tumour |
| ≤10 | 25 (58.14%) | 4 (16.0%) | 21 (84.0%) | 11 (44.0%) | 14 (56.0%) | 3 (12.0%) | 22 (88.0%) |
| >10 | 18 (41.86%) | 6 (33.3%) | 12 (66.7%) | 12 (66.7%) | 6 (33.3%) | 6 (33.3%) | 12 (66.7%) |
| *p-value | | 0.168 | 0.122 | 0.094 |
| Histological grade |
| Low grade | 23 (53.49%) | 8 (34.8%) | 15 (65.2%) | 10 (43.5%) | 13 (56.5%) | 8 (34.8%) | 15 (65.2%) |
| High grade | 20 (46.51%) | 2 (10.0%) | 18 (90.0%) | 13 (65.0%) | 7 (35.0%) | 1 (5.0%) | 19 (95.0%) |
| *p-value | | 0.07 | 0.158 | 0.018 |
| FIGO stage |
| Stage I-II | 16 (37.20%) | 7 (43.7%) | 9 (56.3%) | 4 (25.0%) | 12 (75.0%) | 7 (43.8%) | 9 (56.2%) |
| Stage III-IV | 27 (62.97%) | 3 (11.1%) | 24 (88.8%) | 19 (70.4%) | 8 (29.6%) | 2 (7.4%) | 25 (92.5%) |
| *p-value | | 0.02 | 0.004 | 0.007 |
| Lymph node metastasis |
| Absent | 28 (65.12%) | 10 (35.7%) | 18 (64.3%) | 14 (50.0%) | 14 (50.0%) | 5 (17.9%) | 23 (82.1%) |
| Present | 15 (34.88%) | 0 (0.0%) | 15 (100%) | 9 (60.0%) | 6 (40.0%) | 4 (26.7%) | 11 (73.3%) |
| *p-value | | 0.019 | 0.381 | 0.380 |
| Capsular rupture |
| Absent | 18 (41.86%) | 5 (27.8%) | 13 (72.2%) | 8 (44.4%) | 10 (55.6%) | 6 (33.3%) | 12 (66.7%) |
| Present | 25 (58.14%) | 5 (20.0%) | 20 (80.0%) | 15 (60.0%) | 10 (40.0%) | 3 (12.0%) | 22 (88.0%) |
| *p-value | | 0.405 | 0.242 | 0.094 |
| Lymph/Vascular invasion |
| Absent | 17 (39.53%) | 6 (35.3%) | 11 (64.7%) | 9 (52.9%) | 8 (47.1%) | 6 (35.3%) | 11 (64.7%) |
| Present | 26 (60.47%) | 4 (15.4%) | 22 (84.6%) | 14 (53.8%) | 12 (46.2%) | 3 (11.5%) | 23 (88.5%) |
| *p-value | | 0.127 | 0.599 | 0.069 |
*Chi-square test (χ2) or Fisher’s-exact test; p-value <0.05 to be considered as significant
IMP3: Insulin-like growth factor-2 mRNA binding protein 3; E-cadherin: Epithelial cadherin; FIGO: International federation of gynaecology and obstetrics
Result of Immunohistochemical (IHC) Expression of IMP3 among the Studied 59 Ovarian Serous Tumours
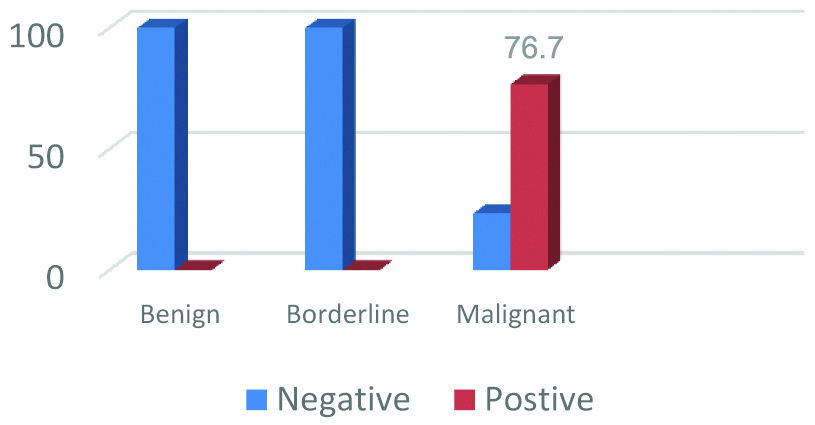
Brown cytoplasmic IMP3 immunoreactivity was detected in 76.7% (33/43) of the studied OSC. Among the ten negative carcinomas, nine carcinomas showed weak to moderate IMP3 staining but in less than 5% of tumour cells, only one was totally negative. 100% (6/6) of the studied borderline tumours were negative for IMP3 (5 cases were totally negative and only one case showed weak staining in less than 5% of tumour cells and also reported negative). Also, 100% (10/10) of the studied cystadenomas were totally negative [Table/Fig-3,4]. Analysis of IMP3 expression with the clinicopathological criteria of the studied 43 OSCs revealed significant expression with advanced FIGO stage (Stage III-IV) and with lymph node metastases (p=0.02 and 0.019, respectively). No, significant association was found between IMP3 expression and other clinicopathological features (p>0.05) [Table/Fig-2].
IMP3 Immunoexpression in the studied 59 Ovarian Serous Tumours.
Representative samples of IMP3 immunoreactivity in ovarian serous tumours: a) Benign serous cystadenoma showing negative staining for IMP3 (x400); b) border line serous tumour showing focal weak staining for IMP3 (x 200); c) low grade serous carcinoma showing weak to moderate diffuse IMP3 staining (x 200); d) high grade serous carcinoma showing strong diffuse IMP3 staining (x 200).

Result of Immunohistochemical (IHC) Expression of EMT Related Markers among the Studied 43 OSC
Membranous E-cadherin immunoreactivity was predominantly lost in 53.5% (23/43) of the studied OSCs and immunoreactive in the remaining 46.5% (20/43) cases. Loss of E-cadherin immunoreactivity was significantly detected in advanced FIGO (III/IV) stage (70.4%, 19/27) OSCs compared to early (I-II) stage (25.0%, 4/16) (p=0.004). Although loss of E-cadherin expression was detected more frequently in high grades (65.0%, 13/20) compared to low grades (43.5%, 10/23) OSCs, but the relationship did not reach significant level (p=0.158). No significant relation was detect between E-cadherin expression and other clinicopathological features (p>0.05). Cytoplasmic Vimentin immunoreactivity (detected in tumour cells, stromal cells, and vessels) was detected in 79.1% (34/43) of the studied OSCs (increased intensity of Vimentin staining was frequently detected at the invasive margin of positive tumours). Analysis of the result of Vimentin expression detected significant expression in high grades (95.0%, 19/20) and advanced stage (III-IV) (92.5%, 25/27) OSCs (p=0.018 and 0.007 respectively). No significant relation was detected between Vimentin expression and other clinicopathological features (p>0.05) [Table/Fig-2,5,6].
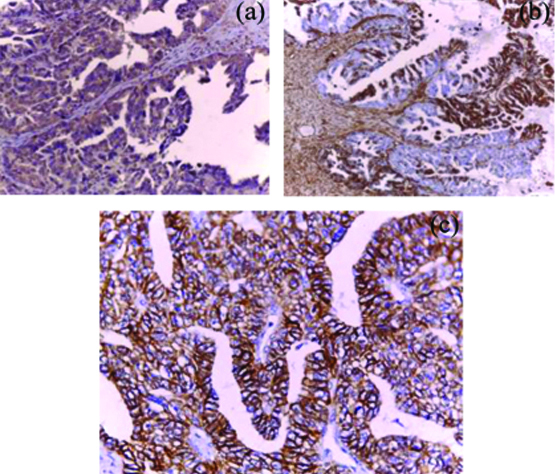
IMP3 and EMT immunoreactivity in low grade OSC. a) Diffuse moderate IMP3 reactivity (x200); b) Focal strong cytoplasmic vimentin reactivity at the invasive margin of tumor cells and stromal component (x100); c) The previous low grade serous carcinoma showing diffuse strong memberanous E-cadherin immunostaining (x400).
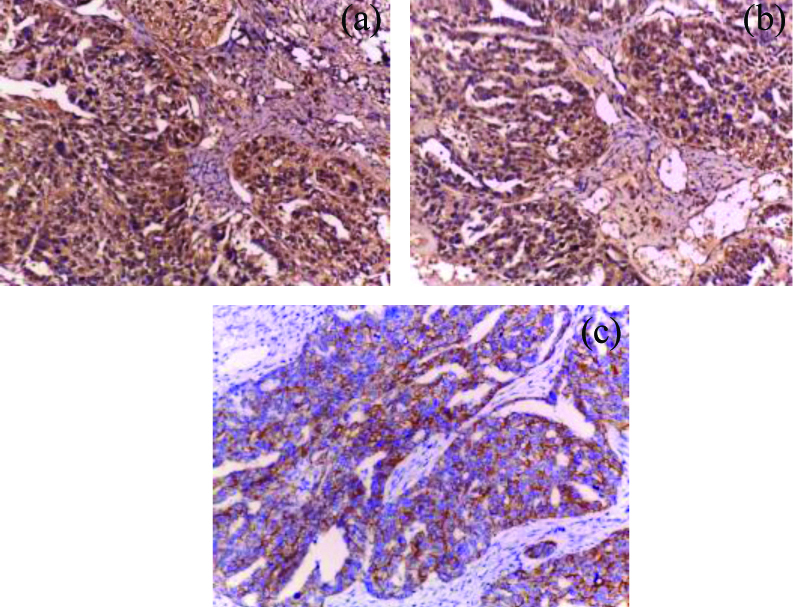
IMP3 and EMT immunoreactivity in high grade OSC. a) Diffuse strong cytoplasmic IMP3 reactivity (x400); b) Diffuuse strong cytoplasmic vimentin immunoreactivity (x400); c) The previous high grade serous carcinoma showing focal weak memberanous E-cadherin immunostaining (x200).
Result of Correlation Analysis between the Expression of IMP3 and the Expression of EMT Related Markers among the Studied 43 OSC
An inverse significant correlation was detected between IMP3 and E-cadherin expressions (Spearman correlation (r)=-0.480, p-value=0.001), while positive significant correlation was detected between IMP3 and Vimentin expressions (Spearman correlation (r)=0.393, p value=0.009) [Table/Fig-7].
Correlation between IMP3 and EMT related markers expression in Ovarian Serous Carcinomas (OSC).
| EMT related markers | n (% of total) 43 (100%) | IMP3 | Spearman correlation value (r) | *p-value |
|---|
| Negative n (% of total) 10 (23.3%) | Positive n (% of total) 33 (76.7%) |
|---|
| E-cadherin |
| Negative | 23 (53.5%) | 1 (2.3%) | 22 (51.2%) | -0.480 | 0.001 |
| Positive | 20 (46.5%) | 9 (20.9%) | 11 (25.6%) |
| Vimentin |
| Negative | 9 (20.9%) | 5 (11.6%) | 4 (9.3%) | 0.393 | 0.009 |
| Positive | 34 (79.1%) | 5 (11.6%) | 29 (67.4%) |
*Chi-square test (χ2)
EMT: Epithelial-mesenchymal transition; E-cadherin: Epithelial cadherin; IMP3: Insulin-like growth factor-2 mRNA binding protein 3
Discussion
Overexpression of IMP3 has been described in several gynaecological and non-gynaecological malignancy [6,7,16]. It is regarded as an essential malignancy specific protein that is linked with aggressive cancers by helping cellular proliferation, invasiveness, and metastasis. [8,16]. Also, EMT have a role in invasiveness and metastatic potential of malignant neoplasms [9,17].
In this study, Authors investigated IMP3 expression in a group of ovarian serous tumours and correlated the expression in malignant tumours with EMT related markers and clinicopathological factors to assess the clinical application of IMP3 in the diagnosis, prognosis and possible application in targeted therapy of such tumours.
We detected IMP3 expression in 76.7% of the studied OSC, while 100% of the studied borderline and benign serous tumours showed negative expression. This is similar to finding obtained by previous related study on serous ovarian tumours [16] and primary mucinous ovarian carcinoma [18]. Findings of the present study that IMP3 is expressed in OSC but not in benign and border line tumours, support the role of IMP3 in ovarian serous carcinogenesis, this is also supported by previous related studies on ovarian [16,18,19], on endometrial [20], on cervical [21] and on gastric carcinomas [7].
Analysis of IMP3 immunoreactivity among the studied 43 OSC, detected significant expression in invasive carcinomas with advanced FIGO stage and with lymph node metastases. This is consistent with the study of Zhu Q et al., who reported significant IMP3 expression in mucinous ovarian carcinomas with invasion-related indexes such as LN metastasis and advanced FIGO stage [18]. Moreover, they reported that IMP3 overexpression facilitated cellular invasion in their in-vitro study. Thus, results of this study support that IMP3 is a prognostic marker associated with advanced stage and tumour invasiveness.
Analysis of EMT related markers expression among the studied 43 OSCs, detected significant loss of E-cadherin expression in advanced FIGO stage carcinomas compared to early stage. Although loss of E-cadherin expression was detected more frequently in high grades than in low grades OSCs, but the relationship did not reach significant level. This is consistent with previous researches that detected loss of E-cadherin immunoreactivity in invasive ovarian carcinomas in comparison with benign, borderline, and well-differentiated ovarian carcinomas [22-25]. Yuecheng Y et al., and Ryabtseva OD et al., detected significant relationship between loss of E-cadherin and clinical stage of epithelial ovarian carcinomas and serous tumours [23,25]. Moreover, they found significant association between E-cadherin loss and tumour grade. In contrast to results of this study, Voutilainen KA et al., and Koensgen D et al., found no significant association between loss of E-cadherin and clinicopathological features of the studied ovarian epithelial cancer [22,24].
The inactivation of E-cadherin gene with the progression of ovarian carcinoma may be attributed to gene mutation, or to two epigenetic mechanisms, including promoter hypermethylation and down regulation transcriptional factors, such as SLUG, SNAI, SIP1, PAK1, and mRNAs [26,27].
Result of present study detected Vimentin expression in 79.1% of the studied OSCs. Analysis of the result of Vimentin expression with clinicopatholgical features detected significant expression in high grades and advanced stage carcinomas. This is consistent with the finding detected by other related researches on ovarian carcinomas [28,29]. Also, Authors detected that vimentin expression with increased intensity was frequently detected at the invasive margin of positive tumours, an observation that was formerly described by Zhi Dong LV et al., [30].
In the present study, correlation analysis between the results of IMP3 and EMT related markers expressions among the studied 43 OSCs, detected an inverse significant correlation between the expressions of IMP3 and E-cadherin and positive significant correlation between IMP3 and vimentin. Therefore, the overexpression of IMP3 contributed to the EMT in OSCs progression. This finding is consistent with the finding of Su P et al., who reported that IHC expression of IMP3 inversely correlated with E-cadherin (r=-0.163, p=0.029) and directly correlated with both SLUG and vimentin expressions (r=0.27, p<0.001 and 0.366, p<0.001, respectively) among the studied breast cancer cases [31]. They concluded that IMP3 could promote invasion and metastasis via EMT in breast carcinoma cells. Jeng YM et al., also reported similar change of E-cadherin expression in IMP3-expressed hepatocellular carcinoma [32]. Thus, results of this study support the evidence that IMP3 is involved in EMT of OSC.
Limitation(s)
The researchers faced some limitations such as financial problems, and limited number of the studied cases due to exclusion of some cases with missed clinicopathological data.
Conclusion(s)
Insulin-like growth factor mRNA-binding Protein 3 (IMP3) is an oncogene unregulated in OSC. It is a prognostic marker associated with tumour aggressiveness. Moreover, IMP3 could promote tumour invasion and metastasis via EMT in OSCs.