Background
Multiple Myeloma (M.M) is a neoplasm of B cell lineage characterized by excessive proliferation of abnormal plasma cells, secreting abnormal immunoglobulin causing monoclonal gammopathy which can be detected by the presence of M protein in serum and urine electrophoresis.
Aim
To detect and quantify monoclonal gammopathy in suspected cases of multiple myeloma and to differentiate them from benign conditions, because of the vast difference between their prognosis and management.
Method
Serum samples from 150 suspected cases of M.M were subjected to serum protein electrophoresis on cellulose acetate strip. M band detected visually and estimation of M protein was done by densitometer. Bone Marrow biopsy and clinical profile were correlated in M band positive cases.
Result
Out of 150 cases 10.66% cases had monoclonal gammopathy. Ten percent cases were diagnosed to be multiple myeloma and one case was found to be Monoclonal gammopathy of undetermined significance.
Conclusion
SPEP is an easy to perform laboratory test which can be used for detection and quantification of monoclonal gammopathy and should be recommended as preliminary test for suspected cases of multiple myeloma. MGUS must be differentiated from M.M, as management and prognosis of these two cases is totally different.
Introduction
Multiple Myeloma (M.M) is a neoplasm of B cell lineage which is characterized by excessive proliferation of abnormal plasma cells. These abnormal plasma cells secrete abnormal immunoglobulin that produces a condition called monoclonal gammopathy, which can be detected by the presence of M protein in serum and urine electrophoresis [1]. It accounts for 10% of the haematological malignancies [2]. It is a debilitating malignancy that is a part of a spectrum of diseases which range from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukaemia. The clinical symptoms that are suspected for a plasma cell disorder include back pain, weakness or fatigue, osteopaenia, osteolytic lesions, spontaneous fractures and recurrent infections [3].
It is very important to distinguish between M.M. from MGUS due to the general nature of manifestation of M.M and the vast difference between the occurrence of M.M. and MGUS. The occurrence of M.M is 4:100000 world wide [4] and that of monoclonal gammopathy of undetermined significance (MGUS) is approximately 1% among the population who are over 50 years of age, it is 3% among those who are over 70 years, and it is up to 10% among those who are over 80 years of age [5–7]. Moreover, the need for the therapy is also very much different in these two conditions. Therefore, serum protein electrophoresis (SPEP) should be done to evaluate the general manifestations like malaise, weakness, chronic bone pain and anaemia, to detect the monoclonal gammopathy and to know the quantity of the M protein in these patients so that we can differentiate between multiple myeloma and the other causes of monoclonal gammopathy.
SPEP is a simple lab technique where the serum is applied on a support medium and exposed to an electric current. The different fractions of the serum proteins separate usually into 5 bands, as – the albumin, α1, α2, β, and the γ globulin fractions. In the interpretation of SPEP, more attention is given to the gamma region, which is mainly composed of Immunoglobulin. Many conditions can cause an increase in the gamma region ,but those which cause a homogenous spike like a peak in the gamma globulin zone, are of special interest. These so called monoclonal gammopathies, result from the proliferation of a single, usually malignant clone of plasma cells which produce either a single class of intact immunoglobulins, heavy chains or light chains or both. These proteins are called para proteins or M(monoclonal) proteins. The M protein or the M component is readily detected as a sharp symmetric spike (M spike) with an α2, β, or a γ mobility while performing the electrophoresis of serum. Multiple myeloma is the most common cause of paraproteinaemia [8,9].
The monoclonal gammopathies include malignant conditions like plasma cell dyscrasias, chronic lymphatic leukaemias and benign idiopathic forms of unknown significance. They may be associated with the drug treatment (Diphenyl hydantoin,sulphonamide and penicillin) [10].
Aim
To detect and to quantify a monoclonal gammopathy by doing SPEP in suspected cases of multiple myeloma.
To differentiate between MGUS and multiple myeloma to facilitate further management.
Materials and Methods
After getting approval from the institutional ethics committee and an informed consent from the participants, 150 blood samples were collected from suspected cases of multiple myeloma and they were subjected to SPEP from Jan 2009 to Jan 2010 in the Department of Pathology, Tata Main Hospital, Jamshedpur. SPEP was performed on cellulose acetate strips by using a ready made buffer (pH 8.6). The cellulose acetate strips were initially soaked in the buffer solution and the extra amount of buffer was removed by placing them in between two Whatman no-1 filter papers. Then, the strips were placed on the central compartment of the electrophoresis chamber. Two filter paper strips were placed on both the sides of the cellulose acetate strip to connect them with the two buffer containing chambers on both the sides of the electrophoresis chamber. Then, 10 microlitre of the serum samples were loaded on the cellulose acetate strip at the sources of the origin. Then, the electrophoresis chamber was connected to the power pack and it was subjected to electrophoresis. After one hour, the strips were removed and they were stained by using Ponceu S. After destaining them by using the reagent which was supplied by the same company, the separated protein fractions could be visualized. The estimation of the individual protein fraction was done by densitiometry (Scannion, Priman Ltd). The M band could be detected visually and the concentration of the M protein was estimated automatically by the densitometer. The buffer, staining reagent and the destaining reagent, all were provided by Priman Ltd. The clinical history and the bone marrow biopsy reports were correlated in the M band positive cases to differentiate M.M from the other conditions.
Result
There were 150 clinically suspected cases of multiple myeloma, whose serum samples were received in the Dept of Pathology for serum protein electrophoresis during the period from Jan 2009 to Jan 2010. There were 90(60%) males and 60 (40 %) females. Among the 150 cases, 16 (10.6%) were found to have monoclonal gammopathy ( the positive M band could be seen in SPEP). The male to female ratio was 1.7:1. A majority of the male patients belonged to the age group of 51 to 60 years, whereas a majority of the female cases were in the range of 61 to 70 years [Table/Fig-1].
Age and Sex Distribution of Cases with Monoclonal Gammopathy
| Age (Years) | Male | Female | Total |
|---|
| 41-50 | 3 | 0 | 3 |
| 51-60 | 5 | 1 | 6 |
| 61-70 | 1 | 5 | 6 |
| 71-80 | 1 | 0 | 1 |
| Total | 10 | 6 | 16 |
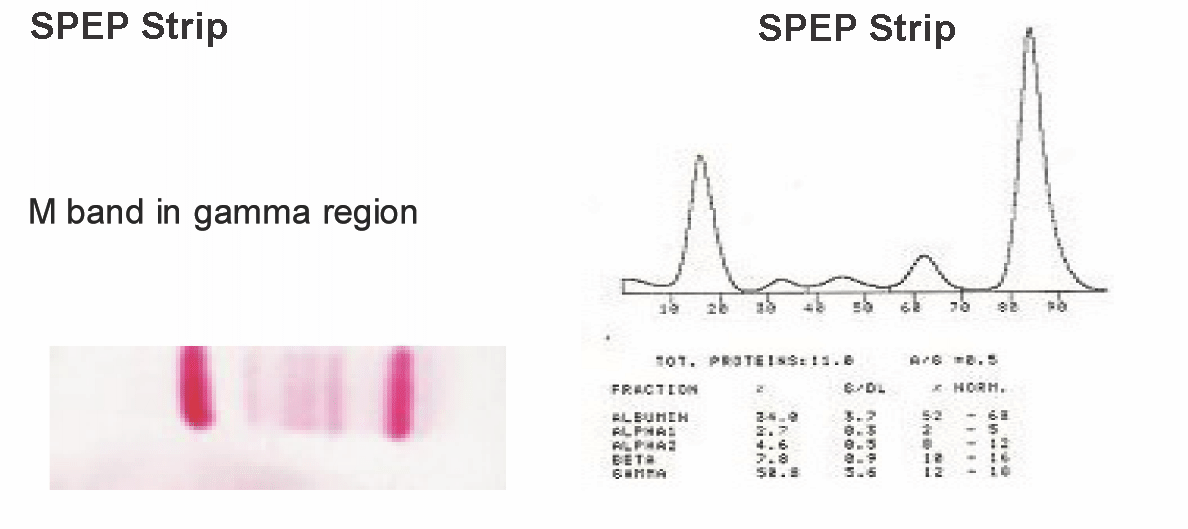
Among these cases, 14 (87.5%) had the M band in the gamma (γ) region and 2 cases (12.5%) had it in the beta (β) globin region. There was no M band in the α region. The mean concentration of the M protein in the γ region was 5.3 g/dl, with a range of 3 to 7 g /dl and in the β region, it was 2.5 g/dl, with a range of 2 to 3 g/dl.
The M spike in the γ region has been depicted on the cellulose acetate strip and in the graph form in monoclonal gammopathy cases in the [Table/Fig-2] and normal SPEP pattern in [Table/Fig-3].
SPEP Strip and Graph showing M spike in gamma region in Monoclonal gammopathy cases
Normal pattern SPEP in our study

Pie chart-showing percentage of different SPEP pattern

The bone marrow biopsy results of the M band positive cases were collected. After correlating the monoclonal gammopathy with the bone marrow biopsy and the clinical features, 15 cases (10%) were diagnosed to have multiple myeloma and one case (0.66%) had MGUS. The percentage of the myeloma cells in the bone marrow was variable and it ranged from 12% to 65% in the cases of multiple myeloma.
Along with paraproteinaemia, the other SPEP patterns which were found in our study were the chronic inflammation pattern, acute inflammation, iron deficiency anaemia and the normal pattern which is show in [Table/Fig-4] by a pie chart.
Discussion
Multiple myeloma, a cancer of the terminally differentiated plasma cells, typically occurs in elderly patients. The prevalence of this disease is low, about 1% of all the cancers, but the incidence increases after the age of 60 years [11]. The term, ‘multiple myeloma’ describes a characteristic feature which is found at multiple sites within the bone marrow (myelo-), with the accumulation of the tumour (oma) cells. Normally, the plasma cells constitute 1% of the cells in the bone marrow, but as the disease advances, the tumour load in the bone marrow increases up to 80%, depending upon the disease severity. These malignant plasma cells synthesize monoclonal antibodies which are released into the circulation. Therefore, the monoclonal protein (antibody) level in the serum. increases [12].
In the clinical practice, serum protein electrophoresis is commonly used to identify multiple myeloma and other serum protein disorders. Many specialists include SPEP as a screening test in the initial evaluation for numerous clinical conditions [13].
In our study, out of the 150 suspected cases of multiple myeloma, 10.6% (16) cases were found to have monoclonal gammopathy or paraproteinaemia, whereas Col. Chopra et al., found 24.4% samples to be positive for the M protein by SPEP [10]. MD Dilawer et al reported 9.2% [14] samples and Vijayashree N reported 4.8% samples to have paraproteinaemia in their studies [15]. Among the 16 M band positive cases that we studied, there was an M spike in the gamma region in 14 (87.5%) cases and 2 cases(12.5%) had an M band in the beta region. Col.G S Chopra et al reported that, 84.8% of the cases had an M band in the gamma (γ) region and that 15.2% cases had an M spike in the beta (β) globin region [10]. In our study, the male to female ratio was 1.7:1, whereas the sex ratio was 1.2:1, as was reported in their study by Col GS Copra et al., [10]. A slight male predominance was also found in the study which was conducted by Nayak B S et al., [16]. The disease manifested in the later ages in females than in the male patients. (between the ages of 51 to 60 years in males and between the ages of 61 to 70 years in females).
Our patients of multiple myeloma fulfilled the criteria for the diagnosis which were given by Durei BG and Kyle RA [17,18]. According to the above criteria, 15 cases (10%) were diagnosed as multiple myeloma in our study, as they had >10% myeloma cells in the bone marrow and >3 g/dl of the M protein in SPEP.
‘Monoclonal gammopathy of undetermined significance’ (MGUS) is a term which was originally coined by the Mayo Clinic group [19]. The incidence of monoclonal gammopathy in the healthy geriatric population is as high as 8% [20]. The patients with MGUS require follow-up, since about 2% develop multiple myeloma or another malignant monoclonal gammopathy per year [21–23]. The diagnosis of MGUS has been a challenge because of its asymptomatic nature and screening for this disease is currently not routinely done in the clinical practice.
The diagnostic criteria for MGUS which were given by the International Myeloma Working Group [24] are as follows-an asymptomatic patient with
Bone marrow which contains < 10% plasma cells
M protein in the serum (<3 g/dl)
No anaemia, lytic lesions or renal insufficiency.
According to this criteria, only one patient (0.66%) in our study was found to have MGUS, who had an M spike in the SPEP, but the concentration of the M protein was < 3 g/dl. There were no myeloma cells in the bone marrow. This patient had an M band in the beta region but he was asymptomatic and he was a follow up case of MGUS. He was 79 yrs old and was the oldest among all the patients.
Conclusion
SPEP is an easy to perform laboratory test which can be used for the detection and the quantification of monoclonal gammopathy. It should be recommended as a preliminary test for the suspected cases of multiple myeloma. MGUS must be differentiated from M.M, as the management and the prognosis of these two cases are totally different.
Limitation
Immunofixation (IFE) is more sensitive than SPEP for detecting the monoclonal immunoglobulins and to identify the heavy or light chain isotype. We could not detect the immunoglobulin isotype due to a lack of IFE in our laboratory.
[1]. Abdalla IA, Tabbara IA, Nonsecretory multiple myelomaSouth Med J. 2002 95(7.) [Google Scholar]
[2]. Breitkreutz I, Lokhorst HM, Rabb MS, Hold B Vander, Cremer FW, Herman D, Glasmacher A, Schmidt wolf IGH, Blan IW, Thalidomide in newly diagnosed multiple myeloma: the influence of the thalidomide treatment on the peripheral blood stem cell collection yieldLaeukemia 2007 21:1294-99. [Google Scholar]
[3]. The International Myeloma Working GroupThe criteria for the classification of the monoclonal gammopathies, multiple myeloma and the related disordersBr J Hematol 2003 121:749-57. [Google Scholar]
[4]. Kyle RA, Multiple Myeloma: An overview in 1996The Oncologist 1996 1:315-23. [Google Scholar]
[5]. Kyle RA, Rajkumar S V, Monoclonal gammopathies of undetermined-significanceHematol Oncol Clin North Am 1999 13:1181-202.PMID: 1062-44 [Google Scholar]
[6]. Kyle RA, Rajkumar S V, Monoclonal gammopathies of undetermined significanceBr J Haematol 2006 134:573-89.PMID: 16938117 [Google Scholar]
[7]. Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, A long-term study on the prognosis of monoclonal gammopathy, of an undetermined significanceN Engl J Med 2002 346:564-9.3rd. PMID: 11856795. [Google Scholar]
[8]. Vavricka SR, Burri E, Beglinger C, Degen L, Serum protein electrophoresis: An underused but very useful testDigestion 2009 79:203-10. [Google Scholar]
[9]. Bottini Verginia P, Laboratory tests for the evaluation of the monoclonal componentRev. Bras. Hematol. Hemoter. 2007 29(1):23-26. [Google Scholar]
[10]. Chopra GS, Gupta PK, Mishra DK, The evaluation of suspected monoclonal gammopathies: the experience in a tertiary care hospitalMJAFI 2006 62:134-37. [Google Scholar]
[11]. Adnan AZ, David HV, Multiple myeloma: an old disease with a new hope for the futureCA Cancer J Clin 2001 51:273-85. [Google Scholar]
[12]. Mehta KD, Khambu B, Lakhey M, Lakhey S, Baral N, Majhi S, The diagnosis of multiple myeloma by the demonstration of the M protein in the bone marrow aspirate, by agar gell electrophoresis: A case reportKathmandu University Medical Journal 2006 4(4, Issue 16):513-16. [Google Scholar]
[13]. O’Connell TX, Horita TJ, Kasravi B, Understanding and interpreting serum protein electrophoresisAm Fam Physician 2005 Jan1(1):105-12. [Google Scholar]
[14]. Dilawar M, liaz A, Hafeez A, Akbar N, Khan FA, The pattern of serum protein electrophoresis in various diseasesPak J Pathol 2005 16(1):22-27. [Google Scholar]
[15]. Vijayashree N, The serum protein electrophoresis pattern in the chronically ill patients in a tertiary care hospitalInd J Clin Biochem 2009 24(supp-):204 [Google Scholar]
[16]. Nayak BS, Mungrue K, Gopee D, Friday M, Garcia S, Hirschfeld E, The epidemiology of multiple myeloma and the role of the M band detection on the serum electrophoresis in a small developing country. A retrospective studyArch Physiol Biochem 2011 Oct117(4):236-40. [Google Scholar]
[17]. Durie BG, The staging and the kinetics of multiple myelomaSeminars in Oncology 1986 13:300-9. [Google Scholar]
[18]. Kyle RA, Multiple myeloma – an overviewThe Oncologist 1996 1:315-23. [Google Scholar]
[19]. Kyle RA, Monoclonal gammopathy of uncertain significance; the natural history in 241 casesMayo Clinic Proceedings 1978 50:29-40. [Google Scholar]
[20]. Boccadoro M, Pileri A, The diagnosis, prognosis, and the standard treatment of multiple myelomaHematol Oncol Clin North Am 1997 11:111-31. [Google Scholar]
[21]. Bergsal D, The incidence and the epidemiology of the plasma cell neoplasmsStem cells1995(suppl-2):1-9. [Google Scholar]
[22]. Ishida T, Dorfman HD, Plasma cell myeloma in unusually young patientsSkeletal Radiol 1995 24:47-51. [Google Scholar]
[23]. George ED, Sadovsky R, Multiple myeloma: recognition and managementAm Fam Physician 1999 59(7):1885-94. [Google Scholar]
[24]. Kyle RA, Rajkumar SV, The criteria for the diagnosis, staging, risk stratification and the response assessment of multiple myelomaLeukemia 2009 January23(1):3-9. [Google Scholar]