Rapidly growing mycobacteria (RGM) are ubiquitous environmental acid-fast bacilli that are characterised by their growth in culture medium within 7 days post-inoculum [1]. M. fortuitum, M. chelonae and M. abscessus are RGM that have emerged as important human pathogens [2]. These emerging infections are increasingly important, especially because of their association with surgical procedures [3].. Rapid growers are usually associated with foreign material. Although they are not found as skin commensals, loss of skin integrity is historically linked to infection [4]. Clinical manifestations include localized abscess formation and chronic ulcers. Diagnosis is often delayed, as Ziehl Neelsen and mycobacterial cultures are not routinely performed on skin biopsy specimens or surgical wound [3].
These atypical mycobacteria are distributed widely in the environment and contaminate municipal water supply and are resistant to sterilizers, antiseptics and standard disinfectants [5]. RGM, the hydrophobic organisms has the ability to form biofilms for its successful survival in the environment. Hence, these organisms are difficult to eradicate even with the regular disinfectants like alkaline glutaraldehydes, organomercurials and chlorine. Shedding of these organisms from the biofilm in a water pipe or device may be the reason for acquiring this infection [6]. Improperly sterilized endoscopes and instruments thus cause post operative wound infections and injection abscess [5].
The frequency of nontuberculous mycobacterial infection is increasing worldwide [4]. In most parts of India there is no standardized operative procedures followed for cultures and also these organisms could be ignored as contaminants, it is not possible to determine the exact incidence of these infections [2].
Herein, we describe case series of surgical site infections due to rapidly growing Mycobacteria seen in our hospital during a period of 12 months as they cause not only physical disfigurement but also emotional distress to the patients, hence awareness of this infection, prompt diagnosis and treatment may ultimately provide better care to patients.
Materials and Methods
The present study included 19 patients (thirteen females and six males, aged 18 – 60 y) who had undergone surgeries like caesarean section, hernioplasty, diagnostic laproscopy, laproscopic cholecystectomy, reduction mammoplasty and presented to Aarupadai Veedu Medical College and Hospital from October 2012 –September 2013. Our inclusion criteria was postoperative wound infections with signs of inflammation of the skin and abscesses or drainage at the wound site in addition to not responding to incision and drainage and antibiotics used for pyogenic infections. Our exclusion criterion was all acute postoperative wound infections of less than seven days from the time of surgery.
Microbiological Investigations
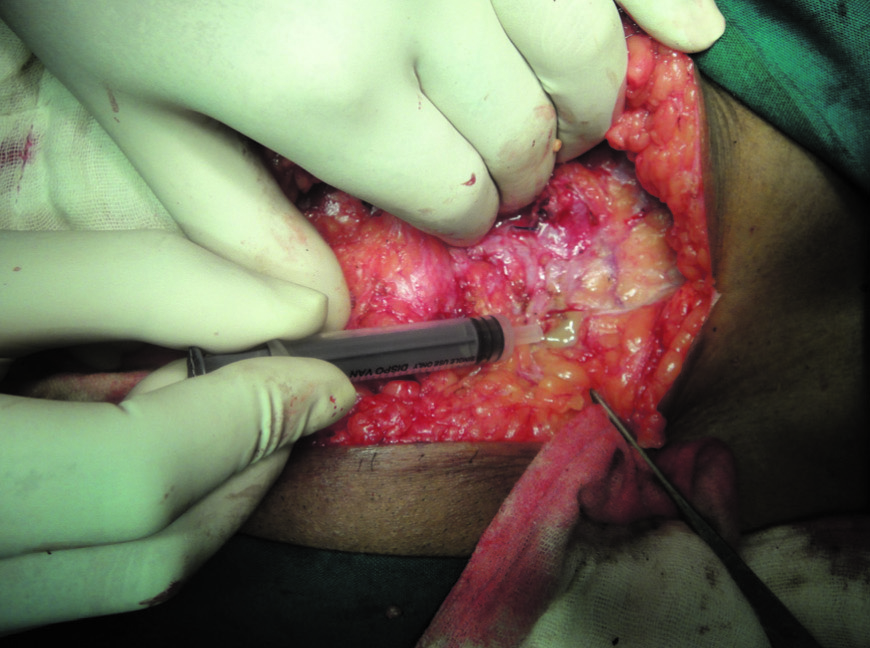
Purulent material of 0.5-2.0 ml aspirate was collected with sterile syringe [Table/Fig-1] or two swabs from the wound site were processed for the identification of the causative agents. In addition in herniotomy patients mesh was also sent. Gram, Ziehl Neelsen and lactophenol cotton blue stains were done for bacteria, mycobacteria and fungi respectively. They were then inoculated in blood, Macconkey, chocolate, Sabourauds dextrose agar and Lowenstein Jensen medium for aerobic bacteria, fungi and mycobacteria respectively. Culture was also put on Robertson cooked meat media to exclude anaerobes. They were incubated at 37°C. The isolated organisms were identified as per the standard bacteriological techniques [7]. The growths on LJ media were further processed for additional tests as shown in [Table/Fig-2] to confirm the identity of the organism. Antibiotic sensitivity testing was done by Kirby Bauer method for amikacin, ciprofloxacin, clarithromycin, rifampicin, tobramycin and linezolid.
Pus being taken from abscess cavity in the anterior abdominal wall
Biochemical tests routinely done to identify mycobacteria
| Test | M.fortuitum | M.chelonae | M.abscessus |
|---|
| Growth at 25°C | + | + variable | + |
| Growth at 37°C | + | + | + |
| Nitrate reduction | + | _ | _ |
| Catalase | + | + | + |
| Aryl sulfatase test 3 days | + | + | + |
| Tolerance to 5% NaCl | + | _ | + |
| Iron uptake | + | _ | _ |
| Beta galactosidase | _ | + | +/_ |
| Tween hydrolysis | + | _ | +/_ |
| Mannitol | _ | _ | _ |
| Sodium citrate | _ | + | _ |
| Inositol | _ | _ | _ |
M.fortuitum, M.chelonae and M.abscessus were identified according to their typical response profiles listed in this table
Results
Nineteen patients with soft tissue infections with RGM were identified. The majority of patients were females (13 females and 6 males) with a median age of 34.7 y (range 18-60 y). All patients had undergone surgery in different surgical settings. The patient had undergone caesarean section (n=6), hernioplasty (n=4), diagnostic laproscopy (n=5), laproscopic cholecystectomy (n=3), reduction mammoplasty (n=1) [Table/Fig-3]. No major disease comorbidities or causes of immunosuppression (e.g., HIV infection) were identified among the patients.
Summary of 19 RGM cases seen in our hospital
| Variable | Values |
|---|
| Age, years {median(range)} | 34.7 (18-60) |
| Sex (n) |
| Male | 6 |
| Female | 13 |
| Type of surgery (n) |
| Caesarean | 6 |
| Hernioplasty | 4 |
| Diagnostic laparascopy | 5 |
| Laproscopic cholecystectomy | 3 |
| Reduction mammoplasty | 1 |
| Incubation period from surgery to symptom onset, days {median (range)} | 42 (20-66) |
| Time interval between clinical presentation and diagnosis, days {median (range)} | 103.9 ( 10-480) |
| Clinical outcome (n) |
| Cured | 16 |
| Not cured no | 2 |
| Lost follow up | 1 |
All the patients were initially treated by repeated incision and drainage, and were started with antibiotics like cloxacillin, second- or third-generation Cephalosporin (such as cefuroxime or ceftriaxone), metronidazole with gentamicin, or amoxicillin/clavulanate as the culture were sterile for routine pyogenic infections done elsewhere. This treatment was repeated on several occasions ranging over periods of one month or more in most cases. The patients presented to our hospital only after the lesions became chronic and recurrent, and no aetiological diagnosis had been established. The median time from surgical procedure to onset of infection was 42 d (range: 20-66 d) and the mean interval between clinical presentation and diagnosis was 103.9 d (range: 10-480 d) [Table/Fig-3].
Skin findings varied widely, including sinus tracts [Table/Fig-4], non healing ulcers, subcutaneous abscesses [Table/Fig-4,5], sub-cutaneous fluctuant or firm nodules of varying size, and erythema [Table/Fig-6] in association with ulcers or chronic drainage from prior surgical wounds. Systemic symptoms such as fever or a chill were absent from all patients.
Erythema, swelling, and abscesses on the abdomen of the female patient around the incision site
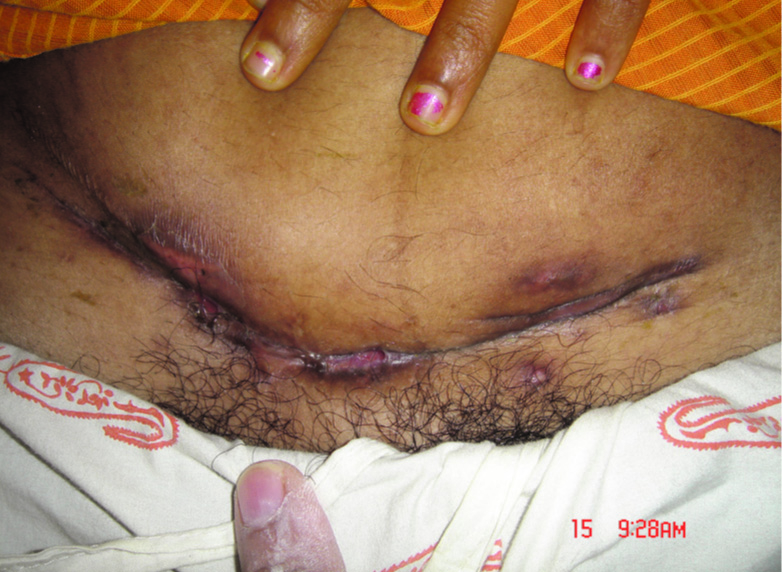
Abscesses, sinuses and scars around the incision site after caesarean section

Erythema with sinus in the wound
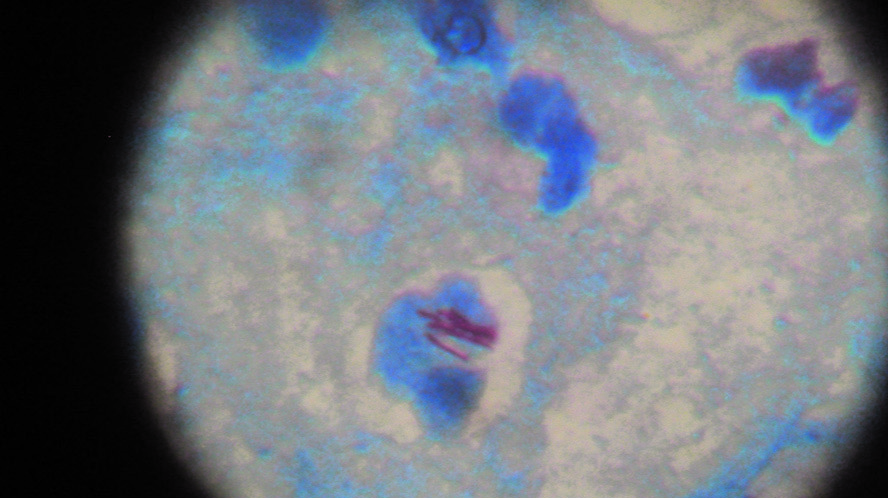
All case-patients had culture-confirmed RGM and also were AFB positive by ZN stain [Table/Fig-7]; species identified were Mycobacterium fortuitum and M chelonae. M fortuitum was also isolated from the mesh of herniotomy patients. Organisms were identified by our laboratory as rapidly growing mycobacteria by standard criteria that included growth rate, morphologic structure, and biochemical test results. Molecular typing of RGM isolates was not performed. All isolates analyzed for antimicrobial susceptibility pattern were sensitive to clarithromycin, linezolid, and amikacin but showed variable susceptibility to ciprofloxacin (82% susceptible), tobramycin (30%susceptible) and rifampicin (58% susceptible).
Ziehl neelsen stain shows acid fast bacilli
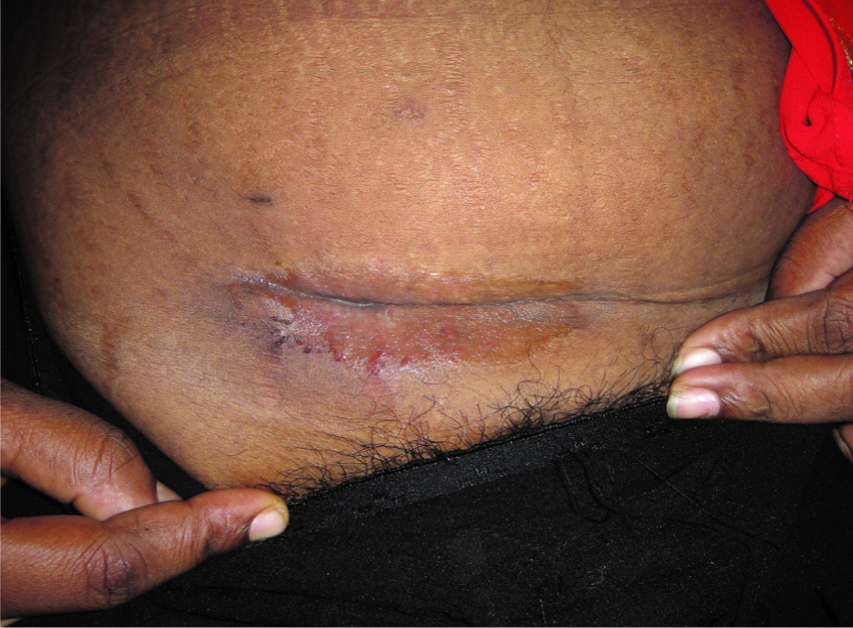
All patients initially underwent surgical procedures to drain existing abscesses and resect any immature nodules. Except the 60-year-old man all were started with amikacin for two weeks and clarithromycin for all the patients. Of the 19 patients 11 patients received clarithomycin for 3 months, 5 patients for 5 months two patient for on and off for 24 months and one patient was lost follow up. In addition for the four herniotomy patients the mesh was also removed. One patient who underwent hysterectomy developed incisional hernia for which hernia repair and excision of the abscess cavity was also done in addition to the antibiotic treatment. All the patients except two and the one who was lost follow up responded well and were cured [Table/Fig-8,9]. Two patients have relapse for two years with repeated incision and drainage and also underwent exploration and drainage under general anaesthesia and yet not cured [Table/Fig-3].
Picture of healed sinuses
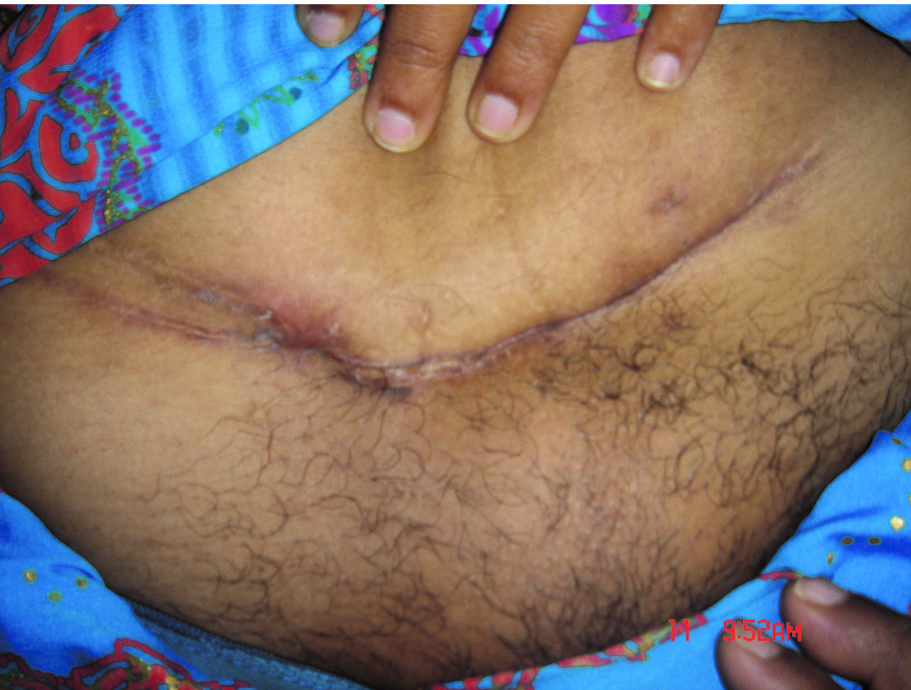
Picture taken after treatment shows healed wound
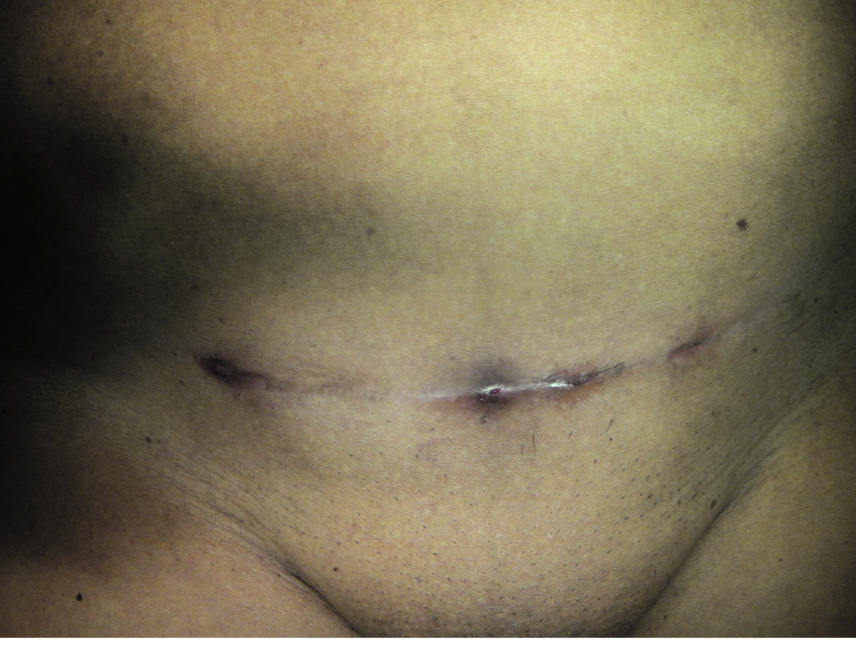
Discussion
Rapidly-growing mycobacteria have emerged as significant human pathogens, causing various infections in healthy and immunocompromised hosts Majority of times, these infections arise following intramuscular injections, surgery, superficial abrasions, penetrating trauma in conditions exposed to contamination of the wound with disinfectants, soil and water solution. These organisms have been increasingly reported in the past three decades in post surgical and post traumatic wound infections and lately increased incidence in localized and disseminated infections including outbreaks due to contaminated instruments have been observed [6].
Postoperative wound infections caused by RGM generally appear some weeks to some months following the procedure [8]. Similarly in our case series the incubation period ranged from 20 to 66 days with a median incubation period of 42 days. On the contrary infections due to other pyogenic bacteria have a shorter incubation period as compared to RGM which have a longer incubation period ranging from several days to several months [9].The absence of clinical response after the administration of antimicrobial agents against commonly invading bacteria (e.g., Staphylococci, Streptococci) and the sterility of routine cultures taken from the infected sites were clues for our infection.
The median time between the onset of symptoms and the microbiological diagnosis was 104 days. Therefore, a high index of suspicion is imperative for the diagnosis to be made. A study by Joon Young Song et al., [9] stated that since the symptoms are relatively mild and indolent, the clinical diagnosis of mycobacteriosis is often delayed and took more than two months from initial manifestation to diagnosis. Also, in the revised literature, most publications conclude that clinical diagnosis of mycobacterial skin and soft tissue infections is not easy to perform and that the diagnosis is often delayed. Delays of more than one year have been reported. A high degree of clinical suspicion and appropriate microbiological techniques are necessary to avoid delays in diagnosis [10].
Clinically the infections caused by RGM in post operative wound infections are similar to pyogenic abscesses with induration, microabscess, and discharge from sinuses and erythema. Systemic manifestations like fever and chills are rare [11]. The clinical features in our study were also similar [12], with erythematous nodules, indurations, microabscesses and discharging sinuses. All our patients presented with only local manifestations that started with painful nodules which gradually increased in size, which then would fistulize and open on the skin draining pus while none of them had any systemic manifestations.
All our patients with postoperative wound infections had repeated sterile aerobic and anerobic cultures. Hence, it is very difficult to clinically diagnose RGM infections because they lack characteristic clinical features. Surgical site infections with abscess and chronic inflammation should be initially managed with conventional antibiotics, however if there is no response and gram stain and routine culture are negative then Mycobacterial infection should be strongly suspected. Hence, AFB staining and culture for Mycobacterium should be done for sterile culture.
The source of infection in our case series is not clear. As per latest article by Maurer et al., a number of sources could have been the possible source of infections which included contaminated gentian violet, rinsing solutions, antiseptic solutions, injectable medications, unsterile surgical instruments or poor wound care, e.g. cleaning postoperative wounds with contaminated tap water [13]. However in case of herniotomy patients in our study as the organisms were also isolated from mesh the source could be either the mesh or the transient presence of the mycobacteria in the surgical environment [4].
In other patients who had undergone caesarean section it is theoretically possible as per other study that nontuberculous mycobacteria might have gained access to the surgical wound from the public water system at the time of showering or it is equally possible that these organisms are present on the skin and are not eliminated by skin preparation preoperatively, thus gaining access through the skin incision [4].
In our current series the source of infection in patients who had undergone laproscopy is primarily attributed to inadequate disinfection of laproscope instruments. The possible reason is; the layer of insulation in the instrument restricts sterilization by autoclave. Moreover, mechanical cleaning of blood and charred tissue that accumulates in the joints of the instruments are also not done properly after surgery. Hence, these contaminated instruments during the surgical process leave endospores on the subcutaneous tissue which germinates and after an incubation period of 3-4 wk clinical symptoms appear [14].
Although many patients had underwent surgery in the same hospital and also under the same environmental conditions it is not clear why only few cases or only sporadic case that also had normal immune systems and no other predisposing medical condition have acquired the infection. Hence, future studies that focus on other conditions that predispose to infection are required.
Successful treatment of RGM requires both surgical treatment and combination of antibiotics [15]. Combination of antibiotics as determined by susceptibility should be prescribed for an adequately long time so that the wound heals and also to ensure that no recurrence occurs. It has been reported that conventional antituberculous drugs are ineffective. Antibiotics should be given based on their susceptibility report and also combination of antibiotics is preferable over single regimen [16]. M.fortuitum responds to antibiotics like amikacin, quinolones, doxycycline and sulphamethaxole. As per latest studies clarithromycin, cefoxitin and imipenem were useful for treatment against RGM [17–19]. Almost all the patients in our study were cured with a combined approach of drainage and clarithromycin based combination therapy. In herniotomy patients mesh was removed. In one female patient who developed incisional hernia as a complication of this infection, underwent hernia repair and excision of abscess cavity.
It has been recommended that to prevent recurrence, antibiotic treatment should be given for a minimum of at least three months, or at least 3 to 6 wk after the wound heals. Recent work has also recommended that antibiotics should be given for 6 to 12 months though the optimal length of treatment has not been yet established [20,21]. Two of our patients had recurrence and had not yet been cured with more than 24 months of therapy, repeated incision and drainage and also exploration under general anaesthesia.
Limitations
The limitations of this paper are as follows: the exact source of infection could not be made out to prevent further infection and also molecular characterization was not done. Since PCR is costly for patients in countries like India identification is still done only by conventional methods.
Conclusion
It is important to re-emphasize the surgeons about the importance of following strict sterilization protocols including cleaning laparoscope instruments as per the manufacturer’s instructions, also proper sterilization of medical equipments, proper skin cleansing preoperatively are essential prerequisite to prevent these infections. Also clinicians should be aware and include RGM in their differential diagnosis of surgical site infections for early diagnosis and prompt treatment.