Androgen levels are found to be low in women infected with HIV. This deficiency may be due to intra-adrenal shunting toward cortisol production and are usually seen in women with significant weight loss. HIV is a highly scrutinized, chronic medical condition with a substantial co-occurrence of mental and sexual health problems [2]. Sexual health problems in HIV infected women have not been extensively studied. Recent studies found out that hypoactive sexual desire disorder (HSDD), pain syndromes and sexual phobias are common among these women [3]. Causes for these disorders can be broadly divided under psycho-social and medical headings [4–6]. Psycho-social causes include grief reactions after developing HIV, anxiety and depression [7], whereas medical causes of sexual dysfunction are low sexual desire secondary to malaise due to advanced HIV disease, endocrinopathies, peripheral and autonomic neuropathies [4–6]. Studies have focused regarding sexual dysfunction among HIV infected men [8,9], however far less is known about such sexual changes among HIV infected women. So, this study intends to understand the relationship between gonadal hormonal abnormalities (free testosterone, FSH and LH) and sexual dysfunction in HIV positive females.
Materials and Methods
Source of Data
All the HIV positive married female patients more than 18 years attending the ART clinic or admitted in the hospital medical wards.
Type of Study
Descriptive/Exploratory study.
Ethical Details
The study was carried out in accordance with the Declaration of Helsinki. Clearance was obtained from the Institutional Ethical Committee before initiation of the study.
Inclusion Criteria
HIV positive married female individuals more than 18 to 45 years.
Denied substance abuse for at least 1 year.
Have not undergone oophorectomy.
Exclusion Criteria
Female HIV individual less than 18 years and more than 45 years.
Patients with sepsis, pregnancy, drug therapy- ketoconazole/steroids.
Patients on gonadal hormone therapy.
Known case of gonadal deficiencies.
Method of Collection of Data
This descriptive/exploratory study was conducted in the Department of General Medicine of a tertiary care hospital from September 2013 to August 2015. A total of 50 patients were taken up for the study. Witten informed consent was obtained from all the HIV positive married female patients attending the ART clinic or admitted in the medical wards. They were also subjected to specific questions regarding sexual dysfunction with the help of female sexual function index by female counselors. Data on the use of anti-retroviral medications was obtained from their medical history and was categorized into protease inhibitors, non-nucleoside reverse transcriptase inhibitors and nucleoside reverse transcriptase inhibitors. Visits of the subjects were scheduled independent of the menstrual cycle. For hormone assay, blood was collected between 8 and 9 am in fasting state. Serum was separated immediately and stored at −20°C. Serum FSH, LH and free testosterone were estimated by radioimmunoassay using PC-RIA. MAS.STARTEC (radioimmunoassay analyser) Startec biomedical system AG, Birkenfeld. Normal reference ranges for the hormonal values were taken as cutoff from laboratory reference range.
Statistical Analysis
The results were analysed using SPSS software (version 18). Parametric tests were analysed using chi-square test whereas non-parametric tests were carried using student’s t-test.
Results
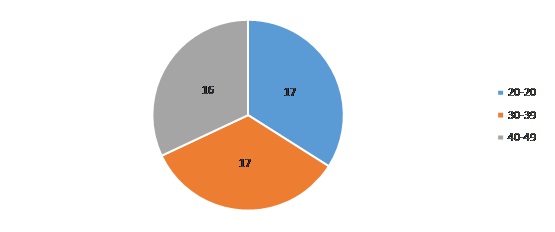
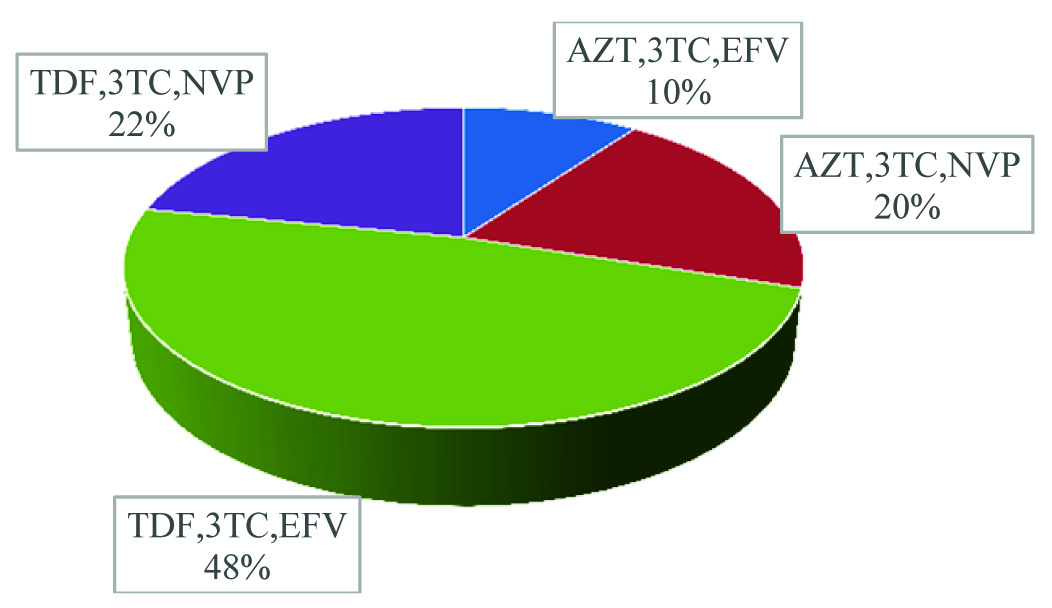
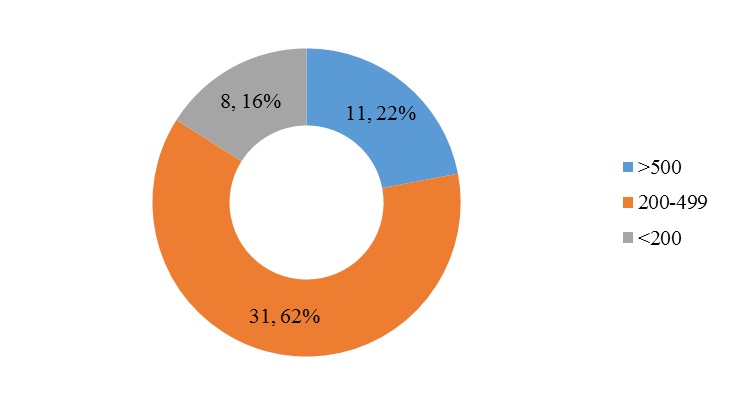
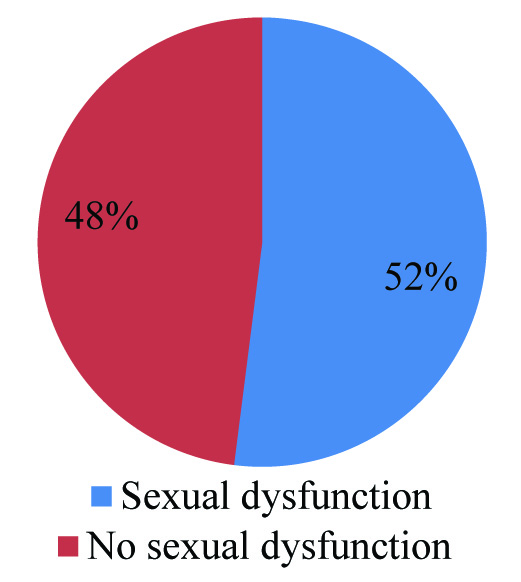
Majority of our study patients belonged to the age group of 20-29 years and 30-39 years [Table/Fig-1] with 48% patients receiving TDF, 3-TC and EFV [Table/Fig-2]. 62% of patients had a CD4 count between 200 and 499 [Table/Fig-3] whereas 52% patients were noted to have sexual dysfunction [Table/Fig-4]. As shown in [Table/Fig-5], the duration of the study was 26 months. Serum gonadal levels were monitored in study subjects [Table/Fig-6]. Concerning sexual functioning, 52% of our sample received a diagnosis of HSSD majority affecting the age group 30-39 years [Table/Fig-7]. We didn’t find any association between drug regimen and sexual dysfunction [Table/Fig-8]. Our study showed significant correlation between CD4 count and sexual dysfunction as majority of the patients in the sexual dysfunction group (18 patients) had CD4 count between 200 and 499 cells/mm3, compared to those with CD4 count less than 200 and above 500 with a significant p-value of <0.02. However the exact reason for this remains unclear [Table/Fig-9]. The results of our endocrine analyses revealed that the hormonal levels of the study patients were in normal range, however the values were in the lower normal range for the study group with sexual dysfunction compared to the non-dysfunctional group. The next question we addressed was whether these endocrine abnormalities affected sexual functioning. We found both free testosterone and FSH, were low in study subjects indicating that primarily the disease was in pituitory rather than in gonads [Table/Fig-10]. It is also conceivable that our failure to control for stage of menstrual cycle or diurnal rhythms may have obscured a true relationship of whether sexual dysfunction was due to primary gonadal disease or primary pituitory abnormality. In our study group we found that duration of HIV is related to sexual dysfunction with a significant p-value of <0.005 [Table/Fig-11]. The scores of each components of FSFI were evaluated and the results are shown in [Table/Fig-12]. As shown in [Table/Fig-13], desire, arousal and lubrication problems were identified in 100% of the patients while orgasm and satisfaction were noted in 60% patients with pain in 52%.
Age distribution of study subjects.
ART status of study subjects.
CD4 count of study subjects.
Sexual dysfunction among study subjects.
Duration of HIV of study subjects.
| Mean | SD |
|---|
| HIV DURATION (months) | 26.14 | 12.46 |
Serum levels of gonadal hormones of study subjects.
| Mean | SD | Minimum | Maximum |
|---|
| Free testosterone | 1.08 | .80 | .08 | 3.01 |
| FSH | 17.29 | 14.91 | 6.57 | 31.87 |
| LH | 20.17 | 9.42 | 5.69 | 47.42 |
Comparison of age and sexual dysfunction.
| FSD Present (P) Absent (A) |
|---|
| No Dysfunction | Dysfunction |
|---|
| Age | n | % | n | % |
| 20-29 | 15 | 88.2 | 2 | 11.8 |
| 30-39 | 4 | 23.5 | 13 | 76.5* |
| 40-49 | 5 | 31.3 | 11 | 68.8 |
*p<0.001.
Comparison of HAART regimen and sexual dysfunction.
| Haart Regimen | FSD |
|---|
| No Dysfunction | Dysfunction |
|---|
| n | % | n | % |
|---|
| AZT,3TC,EFV | 1 | 20.0 | 4 | 80.0 |
| AZT,3TC,NVP | 2 | 20.0 | 8 | 80.0 |
| TDF,3TC,EFV | 14 | 58.3 | 10 | 41.7 |
| TDF,3TC.NVP | 7 | 63.6 | 4 | 36.4 |
p=0.08(Not Significant)
Comparison of CD4 count and sexual dysfunction.
| CD4 | FSD Present (P) Absent (A) |
|---|
| No Dysfunction | Dysfunction |
|---|
| n | % | n | % |
|---|
| >500 | 9 | 81.8 | 2 | 18.2 |
| 200-499 | 13 | 41.9 | 18 | 58.1* |
| <200 | 2 | 25.0 | 6 | 75.0 |
*p=0.02(Significant)
Comparison of gonadal hormonal levels with and without sexual dysfunction.
| FSD Present (P)/Absent(A) | |
|---|
| No Dysfunction | Dysfunction | p |
|---|
| Mean | SD | Median | Mean | SD | Median | |
|---|
| Free Testosterone | 1.51 | .82 | 1.62 | .68 | .55 | .60 | <0.0001 |
| FSH | 20.97 | 20.03 | 17.44 | 13.90 | 6.45 | 11.33 | 0.023 |
| LH | 21.99 | 8.37 | 23.69 | 18.49 | 10.17 | 15.15 | 0.2 |
Comparison of duration of HIV and CD4 count with and without sexual dysfunction.
| FSD | |
|---|
| No Dysfunction | Dysfunction | p |
|---|
| Mean | SD | Median | Mean | SD | Median | |
|---|
| HIV Duration (months) | 21.17 | 9.71 | 24.00 | 30.73 | 13.11 | 24.00 | 0.005 |
| CD4 | 434 | 161 | 451 | 316 | 160 | 309 | 0.01 |
Scores of components of FSFI in study subjects.
| Mean | SD | Median | Minimum | Maximum | Maximum possible |
|---|
| Desire | 2.72 | 1.11 | 2.40 | 1.20 | 4.20 | 6 |
| Arousal | 2.70 | 1.51 | 1.80 | 1.20 | 4.50 | 6 |
| Lubrication | 3.84 | 1.65 | 3.90 | .20 | 5.40 | 6 |
| Orgasm | 3.59 | 1.87 | 3.60 | .00 | 5.30 | 6 |
| Satisfaction | 3.80 | 2.12 | 3.20 | .60 | 6.00 | 6 |
| Pain | 4.14 | 2.20 | 4.00 | .00 | 6.00 | 6 |
| Total FSFI | 20.71 | 9.86 | 18.05 | 3.80 | 31.00 | 36 |
Sexual dysfunction among study subjects for each component of FSFI.
| Sexual dysfunction | n | % |
|---|
| FSD overall | 26 | 52.0 |
| Desire | 50 | 100.0 |
| Arousal | 50 | 100.0 |
| Lubrication | 50 | 100.0 |
| Orgasm | 30 | 60.0 |
| Satisfaction | 30 | 60.0 |
| Pain | 26 | 52.0 |
Discussion
Endocrine dysfunction is common among HIV-infected patients. Adrenal, gonadal, thyroid, bone and metabolic abnormalities have all been reported. HIV itself related infectious organisms, cytokines and antiretroviral medications can all affect endocrine function. Endocrine disorders in HIV disease, for example hypogonadism, adrenal insufficiency, diabetes and bone loss, can cause significant morbidity and are thus important to diagnose. Furthermore, treatment can improve quality of life and long-term mortality [2].
Sexual health is usually mentioned in terms of an individual’s wellbeing. In a HIV infected woman, this is challenged by various social, cultural and economic constraints. Sexual dysfunction affects the sexual functioning in one or more phases of the sexual response cycle [10]. Concerning sexual functioning, 52% of our sample received a diagnosis of HSSD when compared to 39% in Goggin et al., study [11]. This rate was consistent with that found by Brown et al., the other study which systematically diagnosed HSSD in HIV positive woman [12]. With no good estimates on the prevalence of this disorder in the HIV positive or general population, it is hard to know whether this indicates a significantly higher rate than in other populations.
In our study, 52% females had sexual dysfunction and poor grading in FSFI. Similarly a study carried out in Brazil on HIV infected females indentified that about 36% had no sexual arousal until the end of sex act compared to the control group [13]. In 2006, UNAIDS reported that 38% of the Indian women infected with HIV are suffering from various social inequities and property and human rights violations which may predispose them to decreased level of confidence leading to depression and thus sexual dysfunction [14]. Sexual dysfunction is commonly seen in anxiety disorders and in mild, moderate and major forms of depression [15,16].
We informally queried the woman in our study about what they believed had caused a decrease in their sexual desire. The majority of women with HSSD (52%) indicated various reasons, which included fear of rejection, lack of a partner, fatigue, relationship problems and fear of infecting sexual partners. Other issues that may have played a role, but about which we did not systematically enquire were partners’ attitudes about sexual contact and safer sex. In light of our findings, further studies should include a careful analysis of these issues that may play a contributing role in the development of HSDD in HIV positive woman. The results of our endocrine analyses revealed that in our study group the hormonal levels were generally in normal range; however these levels were in the lower normal range in the dysfunction group compared to control group. The next question we addressed was whether these endocrine abnormalities affected sexual functioning. We found both free testosterone and FSH were low indicating that primarily the disease was in pituitory rather than in gonads. It is also conceivable that our failure to control for stage of menstrual cycle or diurnal rhythms may have obscured a true relationship of whether sexual dysfunction was due to primary gonadal disease or primary pituitary abnormality.
Our study showed there was significant correlation between CD4 count and sexual dysfunction since majority of the patients in the sexual dysfunction group (18 patients) had CD4 count of 200-499 cells/mm3, as compared to those with CD4 count less than 200 and above 500 with a significant p-value of <0.02, however the reason remains unclear. Similarly a study in North America confirmed univariate associations between CD4 cell count and sexual function among HIV positive women. CD4+ cell count was correlated with FSFI scores; patients with CD4 ≤199 cells/μL reported lower functioning as compared to those whose cell count was 200 or higher [17]. In our study group we found that duration of HIV is related to sexual dysfunction with a significant p-value of <0.005. We didn’t find any association between drug regimen and sexual dysfunction.
Limitations
Several limitations of the present study need to be mentioned. Besides a relatively small sample, the nature of recruitment may have reduced the likelihood of a representative sample. Future studies might attempt to recruit women from a wider array of service organizations, private doctors, HIV/AIDS testing sites or newspaper advertisements to ensure an unbiased sample. Substance abuse, particularly opiates, is known to affect the endocrine functioning, thus necessitating the exclusion of substance-abusing women from our study. However, the exclusion of these women also limits the generalizability of our findings to HIV infected women who are not actively abusing substances. Our ability to interpret some of the findings would have been improved if we had used a control group. Repeated blood samples taken from the study patients to account for various stages of menstrual and diurnal cycle, rather than a single sample, may have contributed to duplication of information. Further studies are required to consider and evaluate these factors.
Conclusion
In our study group we found that hormonal levels are in normal range, however in the low normal range in the dysfunction group compared to non-dysfunctional group. Both free testosterone and FSH were low indicating that primarily the disease was in pituitary rather than in gonads. We conclude that 100% of subjects had desire, arousal and lubrication problems while orgasm and satisfaction problems were noted in 60% patients with pain reported in 52%. We also conclude that duration of HIV and also level of CD4 count is related to sexual dysfunction. Majority had sexual dysfunction in the age group 30-39. We didn’t find any association between drug regimen and sexual dysfunction.
*p<0.001.
p=0.08(Not Significant)
*p=0.02(Significant)