The discipline of endodontics is governed by paradigms like clinical protocol, quality of instrumentation, effective irrigation, disinfection and obturation of the entire pulp space to achieve a three dimensional seal. The anatomical complexity and variations within the root canal systems enhances bacterial invasion and also makes the cleaning and shaping procedure task oriented. Endodontic instrumentation using both hand and rotary instruments produces organic and inorganic debris that are embedded within a layer of amorphous tissue referred to as the ‘smear layer’. Although the beneficial and detrimental effects of smear layer have plagued various controversies, the influence of smear layer is yet to be established. Presence of smear layer has proven to be deleterious because it prevents the penetration of irrigants, intracanal medicaments and also the filling materials into the dentinal tubules [1, 2]. Despite the presence of controversies, one may deem it prudent to remove smear layer in teeth with infected root canal to allow disinfection of the entire root canal system. Even with the increase in numerous newer irrigants and irrigating devices, the perplexing problem of smear layer removal remains unsolved. Thus, the removal of smear layer demands for combining the efficacy of multiple irrigants, as presently the dissolution of organic and inorganic debris cannot be established with one irrigating solution [2]. One such widely investigated irrigant is super-oxidized water. It is one of most powerful antimicrobial agent available for use in both medical and dental field [3].
The aim of this study was to find a viable alternative to the existing benchmark root canal irrigants with less erosion and clinically acceptable smear layer removal. In the present study, a comparative evaluation was done on the efficacy of EDTA and commercially available super-oxidized water, named Oxum, as a final rinse on smear layer removal and erosion in relation to coronal, middle and apical thirds of radicular dentin using SEM analysis.
Materials and Methods
This in-vitro study was performed in the Department of Conservative Dentistry & Endodontics, Sree Balaji Dental College and Hospitals, Chennai in the year 2012.
Selection of teeth: Freshly extracted 30 human mandibular second premolars indicated for orthodontic extraction were selected. Teeth with straight roots and type I canal anatomy were included in this in-vitro study. All the teeth were radiographed to verify the canal anatomy and the presence for any abnormalities was checked. The extracted teeth were cleaned and were stored in 0.2% sodium azide (Sigma Chemical Co., St Louis, MO) at 40 C.
Canal preparation: The teeth were decoronated using flexible diamond disc to standardize the root length to 15mm and the samples were divided randomly into three experimental groups. The patency and the working length of the canal was determined by inserting #10 K file (Mani Inc., Tochigi Ken, Japan) until it was just visible at the apical foramen (observed under magnifying loupes) by subtracting 1mm from this point. The root canals were cleaned and shaped using Universal Protaper Rotary System (Dentsply-Maillefer, Switzerland) as per manufacturer’s protocol up to F3. Irrigation was performed with 1ml of 2.5% of NaOCl (Ups Hygenies, Mumbai, India) solution after each instrument change. The final irrigation (5 ml) sequence was as follows: Group I-17% EDTA (Pulpdent-Pulpdent Corporation, MA, USA); Group II – OXUM (Sun Pharma, Mumbai, India); Group III - 0.9% Saline (control) (Nirlife, Nirma limited, Gujarat, India) for one minute. All the irrigating solutions were introduced into the canal using stainless steel 27-G beveled needle. The needle was placed within 1 to 2mm of the working length in each canal. Then, the root canals were finally irrigated with 5ml of distilled water to remove any precipitate. The canals were then blot dried with sterile paper points and a sterile cotton pellet was placed and the access cavity was sealed.
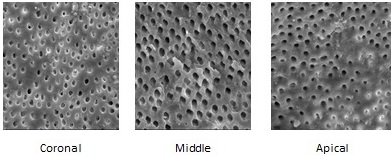
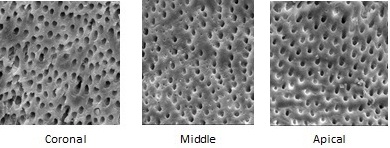
SEM preparation: Using a diamond disc at slow speed, longitudinal grooves were made on the buccal and lingual surfaces of each root without penetrating the canal. The roots were then gently split into two halves using a chisel and were stored in deionized water at 370C until SEM analysis. The specimens were dehydrated using 100% ethyl alcohol and were placed in a furnace at 600C for 24 hours. The samples were then mounted on metallic stubs, gold sputtered using an ion sputter, and examined under scanning electron microscope (LEO 440i, Carl Zeiss, Tokyo, Japan) for the presence or absence of the smear layer. Photomicrographs of the surface morphology at 2000 X magnification of the root canal walls at coronal (10-12mm from apex), middle (6-7mm from apex), and apical (1-2mm from apex) thirds of each specimen were taken. The images were scored according to the following criteria given by Torabinejad et al., [4]:
1 = No smear layer. No smear layer on the surface of the root canal; all tubules were clean and open.
2 = Moderate smear layer. No smear layer on the surface of the root canal, but tubules contained debris.
3 = Heavy smear layer. Smear layer covered root canal surfaces and tubules.
In addition, the degree of erosion of dentinal tubules was scored as follows [5]:
1 = No erosion. All tubules looked normal in appearance and size.
2 = Moderate erosion. The peritubular dentin was eroded.
3 = Severe erosion. The intertubular dentin was eroded and tubules were connected with each other.
These areas were evaluated by two independent evaluators who were unaware of the experimental groups to which the samples belonged.
Results
The SEM images taken of all the experimental groups (coronal, middle and apical third) are shown in [Table/Fig-1,2 and 3]. Mann Whitney results showed that there was no statistically significant difference between the two examiners’ values for scoring the smear layer and erosion in the coronal, middle, and apical thirds for all the tested groups. Kruskal Wallis non parametric tests were used to compare the levels of smear layer and erosion among the groups. Statistical analysis indicated that there is significant difference between coronal, middle and apical third with p-value<0.05, among all the groups [Table/Fig-4]. In group III (saline) heavy smear layer was found in all three regions with no erosion.
Coronal, middle & apical 3rd SEM image of EDTA.
Coronal, middle & apical 3rd SEM image of OXUM.
Coronal, middle & apical 3rd SEM image of saline.

Mean values of smear layer and erosion of all groups.
| Experimental Groups | Mean Score for Smear Layer | Mean Score for Erosion |
|---|
| Coronal3rd | Middle3rd | Apical3rd | Coronal3rd | Middle3rd | Apical3rd |
|---|
| Group IEDTA | 1.33 | 1.53 | 1.83 | 2.83 | 2.63 | 1.93 |
| Group IIOXUM | 1.43 | 2.13 | 2.33 | 2.53 | 1.83 | 1.03 |
| Group IIISaline(control) | 3.00 | 3.00 | 3.00 | 1.00 | 1.00 | 1.00 |
For erosion, in group I (EDTA) showed no statistically significant difference between coronal and middle third, but statistically significant difference was found in apical third as compared to middle and coronal thirds (p-value<0.05). In group II (oxum) statistically significant difference was there between coronal, middle and apical third and it showed significantly less dentine erosion when compared to EDTA.
Discussion
Cleaning and shaping of the root canals is one of the most important phases of endodontic treatment. Literature has shown evidence of smear layer formation routinely over the surface of the dentinal walls after instrumentation [6–8]. The first researchers to describe the smear layer on the instrumented surface of root canals were McComb and Smith. They suggested that the smear layer consists of not only dentin but also the remnants of odontoblastic processes, pulp tissue and bacteria. Mader et al., reported that the smear layer thickness is normally around 1-2 μm [9]. It has been reported that the smear material is made up of two layers: a superficial smear layer and a second layer that is packed into the dentinal tubules. It is present in to the tubules to a depth of about 40 μm [9]. The loosely adherent smear layer can harbor bacteria and provide an entry for leakage structure and hence it should be completely removed from the root canal wall. Despite a variety of irrigating solutions available today, the search for an ideal root canal irrigant is a never-ending problem because of the dentine substrate, smear layer, and the micro-biota within are so complex and resist complete eradication. To effectively remove the smear layer, the most commonly used combination was 17% EDTA with 5.25% NaOCl but the main disadvantages of EDTA is dentinal erosion with limited antibacterial activity [10,11].
In this study, a comparison of the efficacy of smear layer removal by 17% EDTA and a commercially available super-oxidized water (oxum) as a final irrigant was done and the degree of erosion was evaluated. It is evident from the results that the smear layer removal was maximum in the coronal third followed by middle third and it was least in apical third in group I and it was statistically significant when it was compared with group II. It has been shown that both the irrigants were less effective in the apical third. It is due to the stagnation plane of the residual fluid in the apical third as stated by Gulabivala [12].
Vasiliadis et al., reported that dentine in the apical third is sclerosed and that EDTA may not have such a pronounced effect on the apical third as compared to middle or coronal third of the dentine [13]. Michael O Connell et al., compared EDTA of various concentrations and pH and concluded that at high pH, excess number of hydroxyl ion prevented the dissolution of hydroxyapatite crystals thus limiting the number of calcium ions for chelation. Thus at neutral or low pH, the calcium ions from dentine becomes more readily available for chelation due to dissociation of hydroxyapatite crystals [14]. Hulsmann et al., proposed that the ideal concentration of EDTA was from 15–17% with neutral or low pH [15]. At neutral pH, EDTA showed lesser degree of decalcification in the apical third of root dentine because the content of non collagenous proteins decreases in the apical third [15].
In the present study, dwell time of one minute was chosen, which is in accordance with various other studies conducted by Ballal et al., [16]. Also, studies have reported that EDTA when used for more than one minute causes erosion of dentinal tubules, thereby reducing the dentin micro hardness [16]. Saline, which is used as an irrigant in the control group, was found to have no effect on smear layer.
Based on the figures, EDTA had maximum erosion at all three levels of root dentin when compared to the super-oxidized water. The combination of sodium hypochlorite and EDTA offered potential clinical advantages but also produced additional side effects like erosion on the exposed surfaces of the calcospherites. Sometimes the erosion may be so severe to deplete calcospherites completely from the dentine.
Super-oxidized water is a powerful anti-microbial agent against bacteria, fungi, protozoa and viruses. It is rich in reactive oxygen with a neutral pH. The main advantage of this super-oxidized water is that it is stable and has a longer shelf life. It mainly contains oxidized solution (H2O), sodium hypochlorite, hypochlorous acid, hydrogen peroxide, ozone, chlorine dioxide, sodium hydroxide, sodium carbonate and sodium chloride [17]. The molecules are broken into ions and free radicals, which rapidly react and denature protein of bacterial cell wall. It produces an environment of unbalanced osmolarity that damages the cell wall of single cell organisms. The low pH in oxum may sensitize the outer membrane of bacterial cell, thereby enabling oxygen anion radicals to attack the bacterial cell more efficiently [18]. The damage is due to the difference in osmolarity between the concentrations of ions in solution vs the concentration of same ions in the cell [19]. Multicellular organisms are not prone to such changes so host tissues are spared. For these reasons it is referred to as a well suited alternative irrigating agent. Based on the present study, it has shown that oxum when used as an irrigant, cleans the root canal surfaces in a clinically significant manner and removed the smear layer in large areas leaving the collagen fibers intact and completely exposed with less erosion.
Limitation
Routinely the effect of root canal irrigants are evaluated mostly in in-vitro conditions, hence further research to mimic the clinical polymicrobial condition needs to be evaluated.
Conclusion
Within the limitation of the present study, oxum the commercially available super-oxidized water proved to be significantly equal in smear layer removal with less significant erosion when compared to EDTA. However it may be worthwhile to investigate further, the effect of oxum alone as a root canal irrigant to evaluate its effect on smear layer and on dentine.