Early Clinical Implications of Microalbuminuria in Patients with Acute Ischaemic Stroke
Anupa Thampy1, Christopher C. Pais2
1 Postgraduate Student, Department of Internal Medicine, Kasturba Medical College, Mangaluru, Karnataka, India.
2 Professor, Department of Internal Medicine, Kasturba Medical College, Mangaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anupa Thampy, No 63, Thamasha, 4th Main, NGEF Layout, Nagarbhavi, Bengaluru- 560072, Karnataka, India.
E-mail: anupa_thampy@yahoo.com
Introduction
Cardiovascular and cerebrovascular diseases are leading causes of morbidity and mortality worldwide. Stroke accounts for the second leading cause of death, about 11.13% of total deaths worldwide. Microalbuminuria is known to be associated with increased risk of mortality in ischaemic stroke patients. But there have been no studies to assess whether microalbuminuria affects the early clinical outcome of patients with acute ischaemic stroke.
Aim
This study aims to investigate whether microalbuminuria affects the early clinical outcome of patients with acute ischaemic stroke.
Materials and Methods
This is a prospective study of patients with ischaemic stroke (who presented within 24 hours of symptom onset) who were consecutively admitted in three tertiary care centres during the time period from November 2013 to June 2015. Early clinical outcomes in patients were assessed by investigating the presence of Early Neurological Deterioration (END) using the National Institute of Health Stroke Scale. Urine albumin creatinine ratio was divided into two categories – Normal (less than 30mg/g of creatinine) or Urine Microalbuminuria (30-300 mg/g of creatinine).
Results
Total 42 out of 70 patients (60%) were found to have microalbuminuria. In multivariate logistic regression analysis, microalbuminuria was found to be independently associated with END in patients with acute ischaemic stroke (p=0.044).
Conclusion
In the early periods following acute ischaemic stroke, patients with microalbuminuria have worse clinical outcome.
Cardiovascular disease, Cerebro vascular, Early neurological deterioration
Introduction
Management strategies are focused in tackling the increasing burden of cardiovascular and cerebrovascular disease worldwide. In doing so one must approach the risk factors and predictors of these diseases. Microalbuminuria (MA) is a proved predictor and an established risk factor for cardiovascular mortality and morbidity [1–6]. This is not only applicable in diabetic and hypertensive patients but also found to correlate in the general population [2,4].
In addition, various studies have shown MA to be an independent predictor of developing new strokes as well as recurrent strokes and it was associated with increased short term and long term mortality in acute ischaemic strokes [7–10]. Many clinical trials have suggested that albuminuria should not only be considered as a risk assessment marker but a treatment target [2,4].
END in acute ischaemic stroke is a commonly occurring event and has poor long and short term outcomes [11,12]. The predictors of END (Clinical or Radiological), in acute stroke has been repeatedly studied. These outcomes can be improved if predictors can be recognised early and managed appropriately.
In a study conducted by Turaj W et al., where 52 acute ischaemic stroke patients were studied, MA was found in 24 out of 52 (46.1%) acute stroke patients and in 5 out of 37 (13.5%) controls (p<0.05). The 90-day mortality rate was higher in patients with MA as compared to patients without MA (45.8% vs 7.1%) [13].
When Slowik A et al., studied 60 patients admitted within 24 hours of their first ischaemic stroke, MA was found in 46.7% of patients with acute stroke. It was found that patients with MA had a higher mortality than those without MA (21% vs. 3% after 30 days, 39% vs. 6% after 90 days and 50% VS. 9% after 1 year), p<0.05 for all differences [14].
Although MA has been proved to cause increased mortality in stroke patients, there is lack of studies on effect of MA on END. As it is a treatable factor further studies are needed on this aspect.
Objectives of study included:
To find the percentage of patients with acute ischaemic stroke having MA.
To compare the early clinical outcome in patients having acute ischaemic stroke with and without MA.
To determine whether MA is an individual predictor of END.
Materials and Methods
This was a prospective study of patients with ischaemic stroke who were consecutively admitted in three tertiary care hospitals in the time period from November 2013 to June 2015. Patients included were those who had an acute ischaemic stroke and who presented within 24hours of symptom onset. We excluded: 1) Patients who had diseases which would influence the urine protein excretion such as congestive cardiac failure, obstructive uropathy and patients on nephrotoxic drugs (gold, pencillamine, heroin, long term usage of NSAIDs, aminoglycosides) [15]; 2) Patients diagnosed to have urinary tract infection on routine urine analysis or on urine culture; 3) Patients diagnosed to have chronic kidney disease, as this affects the urine albumin excretion; 4) Patients who had fever, severe illnesses and menstruating patients who gave false positives [15]; 5) Patients on treatment with ACE inhibitors or angiotensin receptor blockers.
All the patients with acute ischaemic stroke satisfying the criteria were included in the study. Informed consent was taken from all the patients before taking the history and proceeding with the clinical examination. The following risk factors of stroke were identified:
Hypertension
Diabetes Mellitus (DM) or raised Random Blood Sugar (RBS) on admission
Smoking currently defined as “smoking of cigarette/beedies within the last five years”
A past history of stroke or Transient Ischaemic Attack (TIA).
Neurological evaluation was done with the help of National Institute of Health Stroke Scale (NIHSS) scores on the day of admission and repeated after 24 hours. Computed Tomography (CT) head was done in all patients in the study to confirm the diagnosis and an electrocardiogram was also done. The other investigations that were done for the patients were routine investigations including Complete Blood Count (CBC), Liver Function Tests (LFT), Renal Function Tests (RFT) with electrolytes, Random Blood Sugar (RBS), urine routine and microscopy.
The assessment of MA was based on random morning spot urine done on the first morning after admission in the fasting state. Urine albumin was measured using immunoturbidimetry method and urine creatinine by Jaffe’s method. Urine albumin excretion was estimated as the Urine Albumin Creatinine Ratio (UACR) in mg albumin/g creatinine. The patient’s clinical status was evaluated again on the day of discharge with Modified Rankin Score (MRS).
Statistical Analysis
Collected data was analysed using SPSS for Windows version 17.0. The Fischer-exact-test and Chi-Square test were used to compare categorical variables and Student t-test for continuous variables. Multiple logistic regression analysis was used to evaluate the independent predictors of END. The variables tested were adjusted for age, sex, baseline NIHSS scores and any variable (among the four variables- Hypertension (HTN), Diabetes Mellitus (DM) or raised RBS, Smoking and previous history which was found to have clinical significance) with a p-value of <0.05 was considered significant.
Results
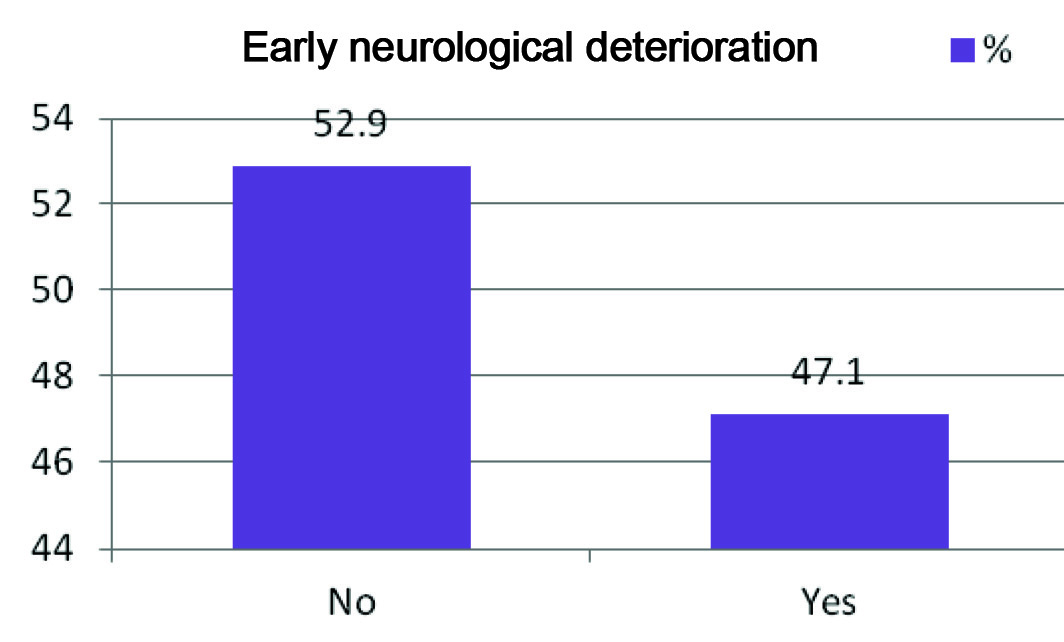
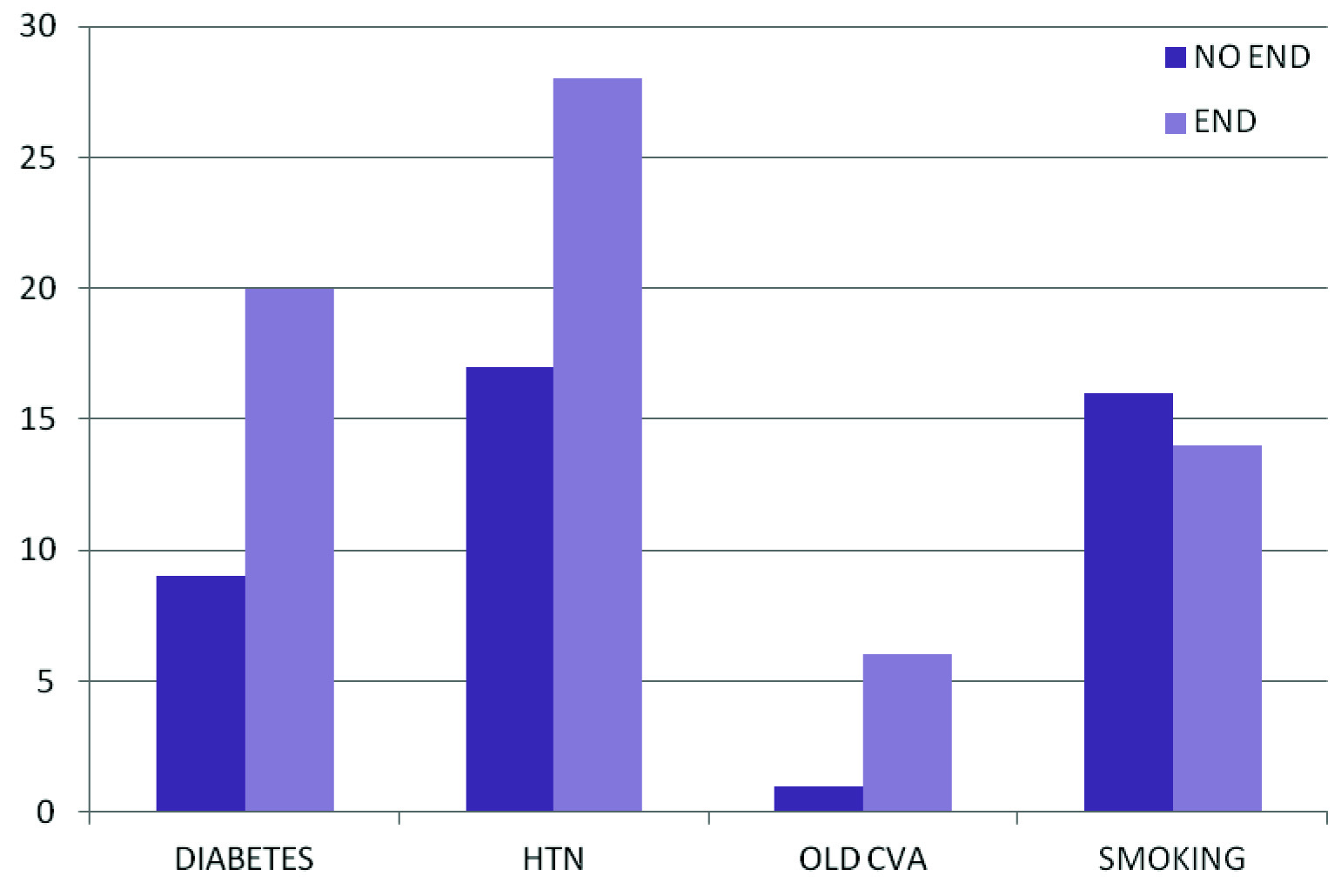
Out of the 70 subjects studied, 33 had END. The prevalence of END in our study was 47.1% [Table/Fig-1]. When we analysed our data, it was found that the subjects predominantly fell in the age group of 61-70 yrs (31.7%) and the mean age was around 62 years. The correlation between age and END was found to be insignificant (p = 0.597). The study subjects were mostly males (72.1%) and there was no significant correlation found between sex and END (p = 0.297). The statistically significant risk factors found in our study were DM, HTN, RBS and history of old Cerebrovascular Accident (CVA) (p=0.002, p=0.001, p<0.001, p= 0.031, respectively). The mean baseline NIHSS score with END was found to be 11.23 and that for without END was 10.55 (statistically insignificant p =0.194). This means that patients with END did not have a higher score in the stroke scale on admission as compared to those without END. The correlation of END with various risk factors has been depicted in [Table/Fig-2].
Prevalence of END.
Y axis: Indicates percentage
X axis: Indicated presence or absence of END
Correlation of END with risk factors of stroke.
Y Axis: indicates percentage
X Axis: Indicates the presence or absence of END in patients with various risk factors for stroke.
Out of the 70 subjects studied, 42 had MA. The prevalence of MA in our study was 60%. Among the 42 subjects 28 (70%) had END and 14 (30%) did not had END. The statistical correlation was found to be highly significant with a p-value of 0.006. Logistic regression analysis which was adjusted for age, sex, baseline NIHSS score, DM, HTN, history of old CVA and RBS showed that MA is an independent predictor of END (p-value = 0.044) as depicted in [Table/Fig-3].
Multivariate regression analysis to predict the Early Neurological Deterioration (END).
| Variables | Logitco-efficient | SE | p-value | Adj.OR | 95%CI |
|---|
| Age in years | -0.089 | 0.052 | 0.096+ | 0.92 | 0.82-1.01 |
| Sex | 2.022 | 1.357 | 0.136 | 7.55 | 0.53-107.9 |
| DM | 0.655 | 1.072 | 0.541 | 1.93 | 0.24-15.73 |
| HTN | 2.434 | 1.211 | 0.044* | 11.41 | 1.06-122.41 |
| CVA | 2.164 | 2.131 | 0.310 | 8.71 | 0.13-566.67 |
| RBS | 0.001 | 0.007 | 0.966 | 1.00 | 0.99-1.01 |
| Micro-albuminuria | 2.753 | 1.365 | 0.044* | 15.69 | 1.08-227.88 |
| NIHSS-Baseline | -0.270 | 0.115 | 0.019* | 0.76 | 0.61-0.96 |
Logit Co-efficient: Logistic Regression Coefficient
SE: Standard Error
Adj. OR: Adjusted Odds Ratio
CI: Confidence Interval
Discussion
Apart from the conventional risk factors for acute ischaemic stroke, recently MA has been found to be a potential risk factor and a predictor of mortality in such patients [7–10].
In our study, the prevalence of END was found to be about 47.1%. The frequency of END differs amongst studies ranging from 12% to 42%. In a recently published Australian study, 19% of acute stroke patients had END and in the Barcelona Stroke Registry, 37% showed END [12]. The probable factor for this difference is that the time frame from symptoms to the first evaluation is varied in different studies.
In the present study the subjects predominately were in the age group of 61-70years (31.7%) and were mostly males (72.1%). There was no significant statistical correlation between END and age (p=0.297) or sex (p=0.597).
Assessment of risk factors of END in our study showed that patients with diabetes, HTN and history of old CVA had increased incidence of END. DM was considered to be a predictor of neurological deterioration in some studies but not in others [16]. In our study, DM didn’t have an individual predictive value of END (p=0.541), where as HTN was found to be an individual predictor of END (p =0.044). This is consistent with the findings in the study conducted by Yamamoto et al., which said that arterial hypertension was an individual predictor of END [16].
Post stroke hyperglycaemia has been found to be associated with lesion expansion as well as poor clinical outcome in both diabetic and non diabetic patients [17–19]. Though our study showed a positive correlation between hyperglycaemia and END (p=0.001), hyperglycaemia could not be considered individually associated with neurological deterioration, when DM and baseline NIHSS scores were taken into consideration (p = 0.966).
Many studies have demonstrated that the initial severity of stroke (Baseline NIHSS value) has a significant contribution to END [12,16]. The results of our study showed that the baseline NIHSS score was not significantly different in the two groups (11.23 in END group vs. 10.55 in non END group), thus not making any significant contribution to neurological deterioration.
MA was found in 60% of the patients, among which 70% of patients had END. The results of our study showed that MA was individually associated with END and thus a higher functional impairment at discharge in acute ischaemic stroke (78.8% of patients with END had MRS at discharge >2).
Some studies have emphasized that MA is an acute phase reactant and cannot be considered as a marker of angiopathy which means that a clinical severe stroke should have an increased urine albumin excretion [20,21]. In our present study, MA was found to be linked to END even after adjusting for the baseline NIHSS score and other risk factors thus signifying that END and MA have a common underlying pathological process.
As previous studies have concentrated only on association of MA with recurrent strokes and long term mortality, our study has a noteworthy contribution to the link between MA and END.
Limitation
The limitations of our study are as follows: 1) Intra-individual variability in MA is a known fact. Urine albumin was only measured in a one spot urine sample in our study. Although one study has shown that the early morning spot-test is equivalent to 24 hours urine albumin measurements [22], it would have been more appropriate to take two other samples; 2) All the potential confounders of urine MA couldn’t be completely ruled out.
Conclusion
In conclusion, MA has been found to be an individual risk factor and it can be used as a marker for predicting which strokes may have END. Management strategies should be focused towards the treatment of MA in acute ischaemic stroke. Further studies are required to clarify the early clinical implication of MA and better treatment options for the same.
Logit Co-efficient: Logistic Regression Coefficient
SE: Standard Error
Adj. OR: Adjusted Odds Ratio
CI: Confidence Interval
[1]. Weir MR, Microalbuminuria and cardiovascular diseaseClin J Am Soc Nephrol 2007 2(3):581-90. [Google Scholar]
[2]. De Zeeuw D, Parving H-H, Henning RH, Microalbuminuria as an early marker for cardiovascular diseaseJ Am Soc Nephrol 2006 17(8):2100-05. [Google Scholar]
[3]. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetesCirculation 2004 110(1):32-35. [Google Scholar]
[4]. Naidoo DP, The link between microalbuminuria, endothelial dysfunction and cardiovascular disease in diabetesCardiovasc J S Afr 2002 13(4):194-99. [Google Scholar]
[5]. Salles GF, Cardoso CRL, Fiszman R, Muxfeldt ES, Prognostic importance of baseline and serial changes in microalbuminuria in patients with resistant hypertensionAtherosclerosis 2011 216(1):199-204. [Google Scholar]
[6]. Stehouwer CD, Smulders YM, Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanismsJ Am Soc Nephrol 2006 17(8):2106-11. [Google Scholar]
[7]. Sander D, Weimar C, Bramlage P, Brandt T, Rosin L, Siebler M, Microalbuminuria indicates long-term vascular risk in patients after acute stroke undergoing in-patient rehabilitationBMC Neurol 2012 12(1):102 [Google Scholar]
[8]. Chen CH, Tang SC, Tsai LK, Yeh SJ, Chen KH, Li CH, Proteinuria independently predicts unfavorable outcome of ischaemic stroke patients receiving intravenous thrombolysisPLoS ONE 2013 8(11):1-6. [Google Scholar]
[9]. Umemura T, Senda J, Fukami Y, Mashita S, Kawamura T, Sakakibara T, Impact of albuminuria on early neurological deterioration and lesion volume expansion in lenticulostriate small infarctsStroke 2014 45(2):587-90. [Google Scholar]
[10]. Chowdhury J, Sultana N, Ahmed S, Rahman MM, Akter M, Rafique T, Microalbuminuria as a predictor of short-term mortality in acute ischaemic strokeBangladesh J Med Biochem 2012 5(1):16-19. [Google Scholar]
[11]. Helleberg BH, Ellekjær H, Rohweder G, Indredavik B, Mechanisms, predictors and clinical impact of early neurological deterioration : the protocol of the Trondheim early neurological deterioration studyBMC Neurol 2014 14:201 [Google Scholar]
[12]. Thanvi B, Treadwell S, Robinson T, Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and managementPostgrad Med J 2008 84(994):412-17. [Google Scholar]
[13]. Turaj W, Słowik A, Wyrwicz-Petkow U, Pankiewicz J, Iskra T, Rudzińsk M, The prognostic significance of microalbuminuria in non-diabetic acute stroke patientsMed Sci Monit 2001 7(5):989-94. [Google Scholar]
[14]. Słowik A, Turaj W, Iskra T, Strojny J, Szczudlik A, Microalbuminuria in nondiabetic patients with acute ischaemic stroke: prevalence, clinical correlates, and prognostic significanceCerebrovasc Dis 2002 14:15-21. [Google Scholar]
[15]. Venkat KK, Proteinuria and microalbuminuria in adults: significance, evaluation, and treatmentSouth Med J 2004 97(10):969-79. [Google Scholar]
[16]. Yamamoto H, Bogousslavsky J, Van Melle G, Different Predictors of neurological worsening in different causes of strokeArch Neurol 1998 55(4):481-86. [Google Scholar]
[17]. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC, Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overviewStroke 2001 32(10):2426-32. [Google Scholar]
[18]. Baird T, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Persistent post-stroke hyperglycemia is independently associated with infarct expansion and worse clinical outcomeStroke 2003 34(9):2208-14. [Google Scholar]
[19]. Lindsberg PJ, Roine RO, Hyperglycemia in acute strokeStroke 2004 35(2):363-64. [Google Scholar]
[20]. Keane WF, Eknoyan G, Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney FoundationAm J Kidney Dis 1999 33(5):1004-10. [Google Scholar]
[21]. Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T, Features of endothelial dysfunction in early diabetic nephropathyLancet 1989 1(8636):461-63. [Google Scholar]
[22]. Teo BW, Loh PT, Wong WK, Ho PJ, Choi KP, Toh QC, Spot urine estimations are equivalent to 24-hour urine assessments of urine protein excretion for predicting clinical outcomesInt J Nephrol 2015 2015:156484 [Google Scholar]