FSGS is a histopathological entity described in a patient who has clinical presentation of heavy nephrotic range proteinuria. Histopathology reveals segmental mesangial sclerosis with/without hyalinosis with adjacent glomerular tuft showing normal appearance on light microscopy. It is imperative that this pathology should be present in absence of any antecedent systemic disease. About 40%-60% of patients with FSGS develop End Stage Renal Disease (ESRD) by the end of 10 to 20 years [1]. FSGS after Renal Transplantation (RT) is known to occur either as a recurrent or de novo lesion. The incidence of de novo or recurrent glomerulopathies occurring in a renal allograft is about 5%-15% and out of these lesions, FSGS is one of the common forms of glomerulopathies encountered in 1%-9% grafts [2-6]. FSGS needs to be addressed because it is one of the important causes of graft dysfunction presenting as proteinuria and progressive graft dysfunction. De novo FSGS is usually diagnosed after one year of transplantation [6-8]. There are many aetiological factors believed to be responsible for de novo FSGS in a renal allograft ranging from CNI toxicity, viral infection, genetic differences or similarity between donor and recipient, hypertension and immunological injury [8,9]. We evaluated RTs of our center to find out the incidence of de novo FSGS.
Materials and Methods
This was a retrospective study of renal allograft biopsies performed between 2007 and 2015. Patient-donor demographics including age, gender, original disease, living/deceased donor, HLA matching, hypertension, induction, immunosuppression, immune injuries and graft function status in terms of SCr and proteinuria were included in study.
Histopathological Analysis
Biopsies indicated for graft dysfunction were included in the study and were evaluated by light microscopy and immunohistochemistry, and reported as per modified Banff 2013 criteria. Morphological evaluation was carried out using Haematoxylin and Eosin (H&E), Periodic acid–Schiff (PAS), Jone’s Methenamine Silver (JMS) and Gomori’s Trichrome (GT) stains on 3 μ thick paraffin sections. C4d antibodies were tested by using polyvalent anti-human C4d antiserum (Biomedical Group, Beckman Coulter, Germany). Diagnosis of histological variants of FSGS was made following Columbia classification [10].
Histological Variants of FSGS were Categorized as Follows
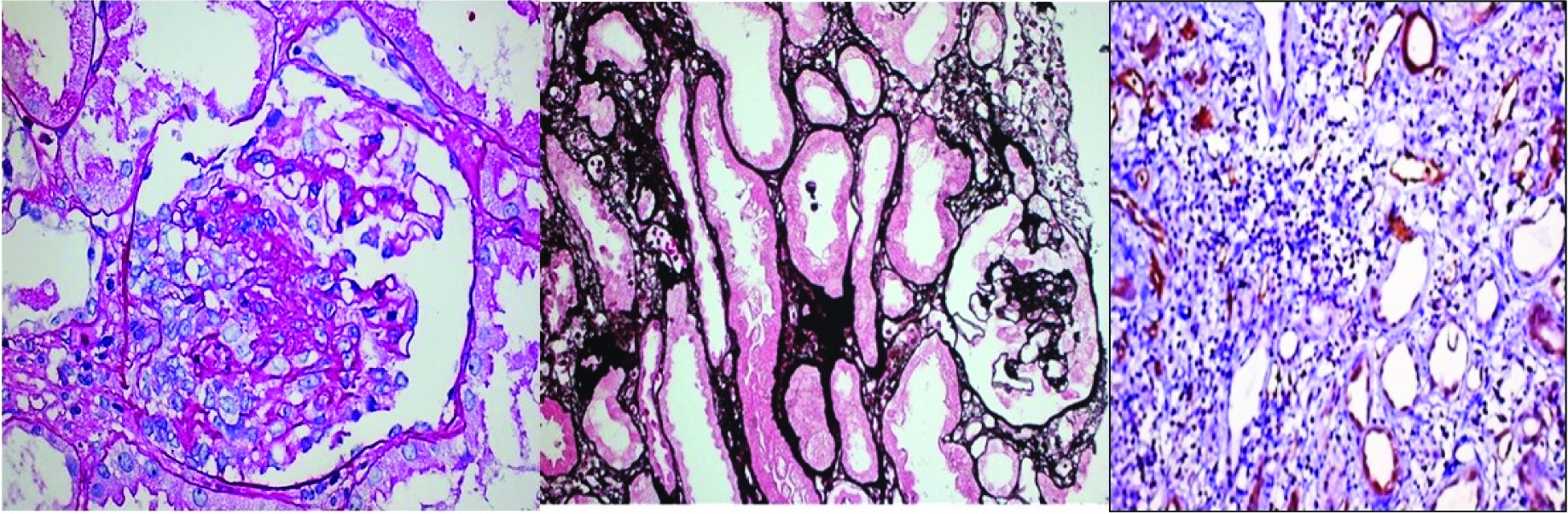
Collapsing glomerulopathy: Segmental and global collapse of glomerular capillaries, wrinkling and retraction of the glomerular basement membrane and marked hypertrophy and hyperplasia of podocytes [Table/Fig-1a-c].
De novo collapsing glomerulopathy with acute T+B cell mediated rejection; a) PAS 40X; b) Jone’s methanamine silver 20X; c) C4d, 20X. (Images from left to right)
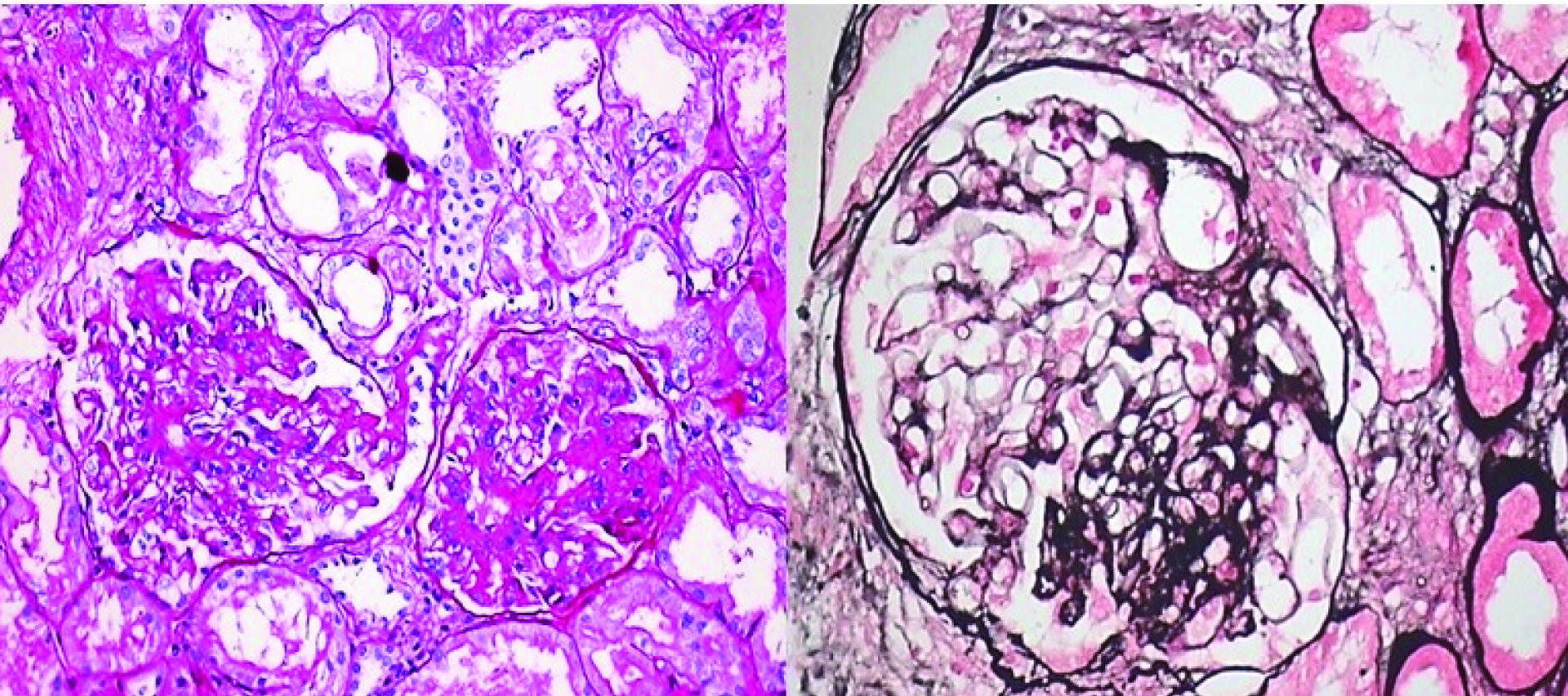
Not otherwise specified (NOS) or classical variant: Focal and segmental consolidation of capillary tuft by increased extracellular matrix, obliterating the glomerular capillary lumen with or without podocyte hyperplasia. Excluding other morphological variants [Table/Fig-2a,b]
Classical (NOS) variant of FSGS: a) PAS 20X; b) Jone’s methanamine silver 40X. (Images from left to right)
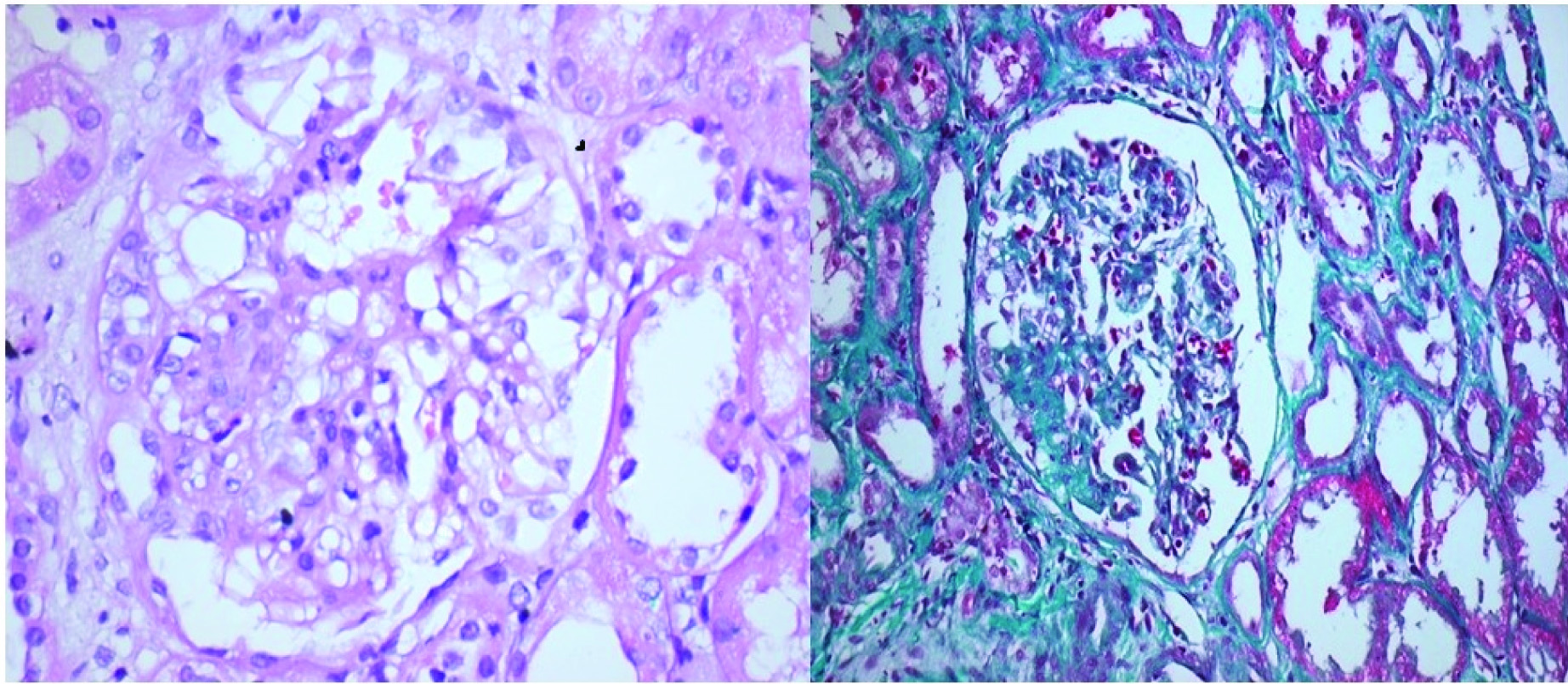
Cellular variant: Only this variant show predominantly endocapillary hypercellularity with occluding capillary lumen with exclusion of tip and collapsing variants [Table/Fig-3a,b].
Cellular variant of FSGS: a) H&E 40X; b) Gomoris’s trichrome 20X (Images from left to right)
Perihilar variant: More than 50% of glomeruli with segmental lesion of perihilar sclerosis or hyalinosis. Excluding cellular, tip and collapsing variants.
Tip variant: Small portion of the glomerular tuft (about 25%) comprising one or two lobules herniated in to proximal tubular lumen with excluding collapsing variant.
Associated Rejections and their Management
All FSGS and associated acute Antibody Mediated Rejection (AMR) were treated with 3-5 cycles of therapeutic plasma exchange (40 ml/kgBW/session) and post-pheresis intravenous immunoglobulins (IvIG), 5 gm/day for five days. Rabbit anti-thymoglobulin, 1.5 mg/kgBW was administered for T-cell rejections (TCR) resistant to 500 mg methylprednisone for three days.
Statistical Analysis
Statistical analysis was performed using SPSS version 20.0 and Microsoft office 2007. Continuous data are presented as mean ± standard deviation. Continuous variables were tested using independent student’s t-test and Mann-Whitney ‘U’-test. Survival analysis was carried out using Kaplan-Meier method with log rank test; p<0.05 was considered to be statistically significant.
Results
Out of 2,599 renal allograft biopsies performed, 42 (1.6%) qualified for de novo FSGS. These belonged to 40 males and two females with mean age of 32.1±10.6 years. Original disease causing ESRD was unknown in 24, hypertensive nephropathy in seven, diabetic nephropathy in three, IgA nephropathy in two, and one each with autosomal polycystic kidney disease, reflux nephropathy, pauci-immune crescentic glomerulonephritis, and obstructive uropathy due to stone disease, sarcoidosis and neurogenic bladder. Majority of the donors were living (n=40), mainly females (n=34) with mean age of 43.8±10.9 years. Donor-recipient HLA match was 2.6±1.2. Donor relationship was parents in 23, spousal in ten, siblings in three and others (including two deceased) in six cases. Mean time of biopsy was 1.1±1.3 years post-transplant and indication was proteinuria of 2.95±2.87 grams/24 hours with raised SCr of 2.24±0.26 mg/dL.
Histopathology revealed collapsing variant in 20 (47.6%), NOS/ classical variant in 15 (35.7%), cellular variant in four (9.5%), perihilar variant in three (7.1%) biopsies. Associated AMR + TCR were observed in 15 (35.7%) biopsies. Five biopsies revealed acute on chronic CNI toxicity.
All the patients were on anti-hypertensive therapy consisting of angiotensin receptor blockers or calcium channel blockers. Majority (52.4%) of the patients were on CNI based immunosuppression with 4 on cyclosporine, 3 mg/kg BW/day, 18 on tacrolimus, 0.05 mg/kg BW/day and two on sirolimus, 2 mg/day as the principal immunosuppressants. Remaining patients (42.9% i.e., 18) were on mycofenolate, 360 mg thrice a day and prednisone, 5-10 mg/day.
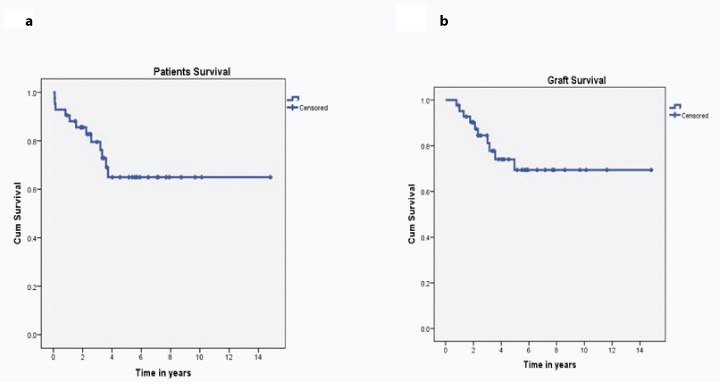
Nearly 12 (28.6%) patients and 10 (23.8%) grafts were lost over a mean follow-up of 2.40±1.30 years, as depicted in Kaplan Meier chart [Table/Fig-4a,b]. Mean SCr of the remaining 20 (47.6%) patients was 1.98±0.67 mg/dL.
Kaplan Meier chart of patient and graft survival.
Discussion
Post-transplant glomerular disease is a frequent complication of RT that may worsen the graft function and impair long term patient survival. The incidence rate of post transplant glomerulonephritis was 9.7% and 17% at five and 10 years of follow up, respectively [3]. De novo GN in RT recipients is rarely encountered. Incidence of de novo FSGS is 1%-9% in renal allografts according to various studies [2-5]. Study by An JN et al., documented an incidence of 8.2% [3]. Our study of 2,599 renal allograft biopsies evaluated over a period of nine years shows an incidence of 1.6% of de novo FSGS. Recurrent FSGS usually presents in early post-transplant period with nephrotic syndrome whereas de novo FSGS present after one year of RT with variable amount of proteinuria, hypertension and progressive deterioration of renal allograft function [2-9]. The mean duration of development of de novo FSGS in our study is 1.1±1.3 years post-transplant.
The pathogenesis of de novo FSGS in renal allograft is unknown but two potential pathogenic mechanisms like CNI toxicity and hyperfiltration are implicated. Compensatory glomerular hyperfiltration in residual nephrons caused by nephron loss/low nephron dose in condition like adult recipients of paediatric kidneys or small size donors with large body mass index of recipient. Sometime long standing grafts with nephron loss and fibrosis resulting in to hyperfiltration injury [11,12]. The glomerular hypoperfusion in grafts with severe vascular disease resulting in to subsequent collapsing glomerulopathy. CNI increased vasoconstrictor factors like endothelin, thromboxane and also activate renin angiotensin system, as well as reduce vasodilator factors like prostacyclin, prostaglandin E2 and nitric oxide. Both these factors leads to arteriolar hyalinosis, vasoconstrictor and reduce GFR. CNI can increase the expression of TGF-β in podocytes may lead to podocyte apoptosis and detachment from the glomerular basement membrane leading to synechia and glomerulosclerosis [13,14]. Hypertension, Diabetes Mellitus (DM), immunological injury [6], BK polyoma virus or Parvo virus B19 infections [15,16] and any other condition leading to loss of renal mass can be involved in pathogenesis of FSGS. Sirolimus have a direct toxic effect on podocyte which ultimately leads to decreased Vascular Endothelial Growth Factor (VEGF) expression in podocytes and develop FSGS [17,18]. Our study with 52.4% patients on CNI based regimens, also confirms the role of CNI in causation of de novo FSGS. Hypertension was the strongest associated finding in our patients, with 100% patients on anti-hypertensive agents. Similarly, living-related donors were the second most significant association with de novo FSGS in our study reflected by 92.3% patients having related donors. This finding again favors the concept that deceased or HLA-mismatched RTs should be performed to avoid occurrence of de novo FSGS.
Perihilar and NOS variants are more common in de novo FSGS [6]. Gupta R et al., have noted an incidence of NOS variant in 70.3%, perihilar and collapsing each, in 13.5%, and tip lesion in 2.7% biopsies [8]. Our study shows that collapsing variant is the commonest morphological form of de novo FSGS observed in 47.6%, followed by NOS variant in 35.7%, cellular variant in 9.5% and perihilar variant in 7.1% of the biopsies.
Human Immunodeficiency Virus (HIV) is the most commonly recognized cause of collapsing variant of FSGS, but non-HIV associated collapsing variant is also well-described in both native kidneys and renal allografts [19,20]. Meehan SM et al., have observed collapsing variant in about 3.2% of RTs [21].
Prognosis of FSGS post-transplant is poor, with a survival of only 40% at five years after diagnosis in cases associated with chronic allograft nephropathy [1,8]. Collapsing glomerulopathy in renal allograft biopsies is associated with more severe vascular changes in form of arteriolopathy as compared to other variants of FSGS, higher degree of proteinuria, renal insufficiency and higher rate of graft loss. In a study by Swaminathan S et al., all patients lost their grafts within three years of diagnosis of de novo collapsing glomerulopathy [22].
Graft loss develops very quickly in recurrent FSGS as compared to de novo FSGS and these patients return to dialysis earlier as compared to patients with de novo FSGS which has slow progression. In our study also 28.6% patients and 23.8% grafts were lost at the end of 2.4 years. However, in the remaining 47.6% patients who survived with functioning grafts by the end of nine years post-transplant, the mean SCr was 1.98±0.67 mg/dL.
It is very important to identify the factors/aetiologies responsible for FSGS post-transplant for early therapeutic intervention for better graft and patient survival. Close follow-up with urinary protein estimation and SCr will help in improving the outcome of RT and avoid graft loss due to recurrent or de novo FSGS. However, biopsy still remains the gold standard for the diagnosis. Simultaneous immunological injury and FSGS can also occur in a renal allograft as reflected with the incidence of 37.5% rejections in our study suggesting the role of possible common immune dysfunction in the form of loss of T- and B-regulatory cell functions which needs to be studied in future.
Limitation
It is believed that with more third party transfusions, and more number of dialysis associated with longer waiting period chances of FSGS decrease. Donor-recipient size mismatch are known to lead to hyperfiltration increasing the susceptibility to develop FSGS. We have not been able to procure the data related to maintenance hemodialysis procedures, third party transfusions, and donor-recipient size mismatch in our study to throw more light on the subject.
Conclusion
De novo FSGS can occur after the first year of RT especially with related HLA-matched donors leading to eventual graft loss inspite of adequate treatment. Hypertension and proteinuria indicate possibility of impending FSGS in these patients.